A Fatal Twist in Pseudohyperkalemia
Devera JL, Barnes DK, Lewis WR. A Fatal Twist in Pseudohyperkalemia. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Devera JL, Barnes DK, Lewis WR. A Fatal Twist in Pseudohyperkalemia. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
The University of Maryland School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. This activity is jointly provided by the Agency for Healthcare Research and Quality (AHRQ) PSNet.
Debra Bakerjian, PhD, APRN, RN; David K. Barnes, MD, FACEP; Noelle Boctor, MD; Justin L. Devera, MD; William R. Lewis, MD; Patrick Romano, MD, MPH for this Spotlight Case and Commentary have disclosed no relevant financial relationships with ineligible companies related to this CME and CPE activity.
Learning Objectives
At the conclusion of this educational activity, participants should be able to:
- Recognize ECG manifestations of hypo- and hyperkalemia
- Recognize causes of in vitro hemolysis
- Describe treatment strategies for torsades de pointes
The Case
A 54-year-old man with a history of tobacco use presented to the emergency department (ED) with a chief complaint of acute chest pain. The pain was intermittent, began 2-3 weeks prior, lasted several minutes to hours in duration, and radiated to his left arm. The patient thought the pain was related to his diet and put himself on a juice cleanse one week before his presentation. On arrival at the ED, he was afebrile, heart rate was 98 beats per minute (bpm), blood pressure was 103/87 mmHg, and oxygen saturation was normal on supplemental oxygen. On physical examination, the patient had bibasilar rales and 1+ bilateral lower extremity edema. His 12-lead electrocardiogram (ECG) demonstrated normal sinus rhythm and right bundle branch block (RBBB). There was also prolongation of the QT interval, flattened T waves, and small U waves that were not recognized by either the machine or the ED physician. Laboratory tests were notable for hyponatremia to 126 mEq/L, hyperkalemia to 5.9 mEq/L, elevated creatinine to 1.4 mg/dL, moderately elevated troponin, and normal serum magnesium and calcium. The lab sample was reported as hemolyzed, but this finding was not recognized by the treating physician. Chest x-ray revealed mild cardiomegaly and pulmonary vascular congestion.
The patient subsequently developed atrial fibrillation with rapid ventricular response at approximately 120 bpm. Cardiology was consulted and recommended initiation of heparin, amiodarone, and metoprolol. The patient also received treatment for hyperkalemia with albuterol, intravenous calcium, insulin with 50% dextrose, sodium bicarbonate, and furosemide. Soon after receiving these medications, he became unresponsive and pulseless. Torsades de pointes (TdP) was present on telemetry, though this was interpreted as ventricular fibrillation (VF) and treated with epinephrine, amiodarone, and sodium bicarbonate. Despite routine resuscitative efforts, return of spontaneous circulation was not achieved. Autopsy confirmed sudden cardiac arrest without myocardial infarction as the cause of death.
The Commentary
By Justin L. Devera, MD, David K. Barnes, MD, FACEP, and William R. Lewis, MD
This case illustrates how the misinterpretation of a common laboratory complication can lead to incorrect treatment and patient harm. Several errors led to the poor outcome, stemming from an initial failure to recognize pseudohyperkalemia due to hemolysis. Unfortunately, the patient deteriorated and suffered sudden cardiac death likely due to TdP (meaning “twisting of the points” in French), a type of polymorphic ventricular tachycardia (VT) characterized by waxing and waning QRS amplitude. TdP was likely precipitated by severe hypokalemia in the setting of a prolonged QT interval and then degenerated into VF.1 The case exemplifies how a poor patient outcome is often the product of a series of errors (both human and technical), and why it is important to mitigate cognitive biases and optimize clinical workflows to improve patient outcomes.
The Impact of Hemolysis
In vitro hemolysis is common, contributing to approximately 40% of all laboratory errors and affecting up to 12% of all samples sent to clinical laboratories, especially in busy clinical settings such as the ED.2–4 Hemolysis is defined as a generalized process of injury to blood cells and can be due to biological factors (e.g., intravascular hemolysis) or non-biological conditions during the collection and handling of blood samples.2 Examples of the latter include traumatic venipuncture, sampling from intravenous catheters or through very small needles, vigorous shaking of blood samples, and long-distance transportation with improper storage.2 Hemolysis releases intracellular components into the serum thereby spuriously increasing the concentration of analytes such as potassium (i.e., pseudohyperkalemia).2 In one retrospective study, the mean difference in potassium concentration between in vitro hemolyzed samples and non-hemolyzed samples was 1.9 mmol/L, with a minimum and maximum difference of 0.7 and 4.6 mmol/L, respectively.3
The Role of Potassium
Potassium is an intracellular cation with a critical role in the activity of the sodium-potassium (Na+-K+) ATPase transmembrane energy pump. In cardiac myocytes, the high selective permeability of potassium ion channels in the cell membrane generates a negative resting membrane potential that stabilizes cardiac myocytes.5 Therefore, serum potassium levels are closely regulated physiologically to maintain values between 3.5 and 5.0 mEq/L. Hypokalemia, defined as serum potassium <3.5 mEq/L, can be caused by decreased intake, renal and gastrointestinal losses (e.g., diuretic therapy, diarrhea), or transcellular shifts.6 ECG manifestations of hypokalemia include ST-segment depression, dynamic T wave changes with flattening or inversion, U waves, and prolongation of the QT interval (Figure 1).6,7
Hypokalemia leads to resting membrane hyperpolarization, inhibition of the Na+-K+ ATPase pump, and decreased potassium channel conductance.5 These changes result in cardiac action potential prolongation, which increases the risk of arrhythmia through various mechanisms, such as reduced repolarization reserve, early after depolarizations (EADs), delayed after depolarizations, and abnormal automaticity.5 These mechanisms provide the substrate for ventricular arrhythmias, including polymorphic VT, TdP, and VF, which are propagated by calcium and sodium currents and maintained by reentry.5 Hypokalemia may potentiate the effects of class III antiarrhythmic drugs, such as amiodarone, suppressing outward potassium currents and thereby lengthening the action potential, prolonging the QT interval, and increasing the risk of TdP.5,8
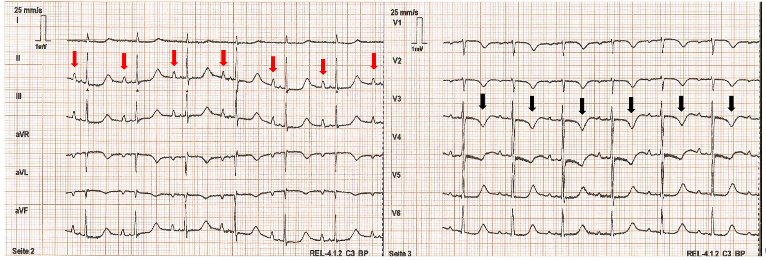
Figure 1 – ECG Demonstrating Hypokalemia. In this 12-lead ECG, hypokalemia is evidenced by prominent U waves (red arrows) and T wave inversions (black arrows). Image adapted from Wikimedia.
On the other hand, hyperkalemia is defined as serum potassium >5 mEq/L in adults and can produce ECG changes at serum concentrations as low as 5.5 mEq/L.6,9 The earliest ECG manifestation is an increase in T wave amplitude, with the appearance of “peaked” T waves (Figure 2).9 When hyperkalemia is severe (i.e., >6.5 mEq/L), flattened P waves, prolongation of the PR interval, and widening of the QRS can also be seen.9 At the most extreme levels of hyperkalemia, a “sine wave” pattern produced by the fusion of a widened QRS complex and T wave can develop, and arrhythmias such as sinus bradycardia, sinus arrest, VT, VF, and asystole may occur.6,9 Treatment of hyperkalemia is generally reserved for patients with associated symptoms (e.g., muscle weakness, paresthesias, or palpitations), ECG changes, rapid-onset or severe hyperkalemia >6.5 mEq/L, or underlying heart disease, cirrhosis, or renal dysfunction.6
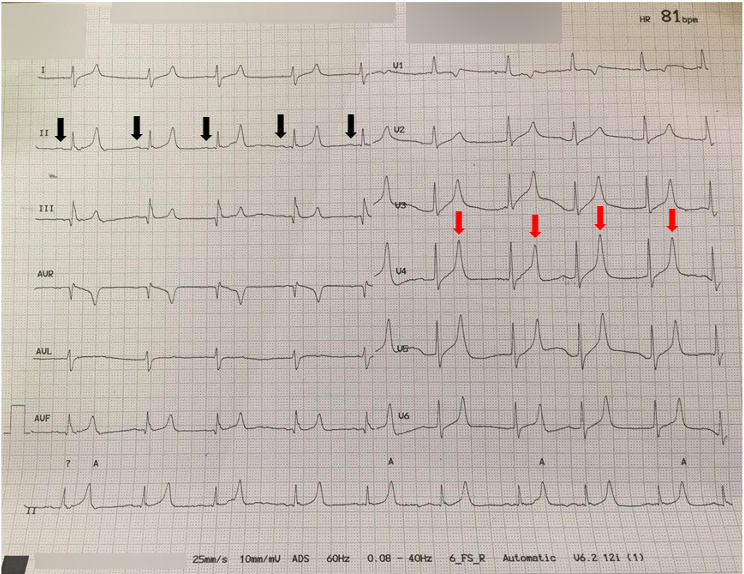
Figure 2 – ECG Demonstrating Hyperkalemia. In this patient with a serum potassium level of 8.2 mEq/L, peaked T waves (red arrows) and flattened P waves (black arrows) are evident. Image adapted from Wikimedia.
Unwinding the Case
Despite the laboratory report of hyperkalemia, the patient presented with several signs suggesting the opposite – hypokalemia − including T wave flattening, U waves, and prolonged QT interval (although the QT interval may have been overestimated to some extent by the presence of RBBB). These findings could have prompted the physician to question the validity of the lab measurement. Even if the ECG signs of hypokalemia were not appreciated, the presence of RBBB should have prompted a review of prior ECGs to assess for any new findings and/or repeating the ECG to evaluate for any dynamic changes. Moreover, a serum potassium level of 5.9 mEq/L does not typically warrant aggressive treatment in the absence of hyperkalemia-related symptoms, ECG changes, or arrhythmias, as described above.
The patient presented with all the ingredients of a “perfect storm” for TdP – hypokalemia exacerbated by transcellular shifts (from albuterol, insulin, and sodium bicarbonate), renal loss from furosemide, and the effects of a class III antiarrhythmic/QT-prolonging agent (amiodarone) that can increase risk of TdP in the setting of an already-prolonged QT interval and acute decompensated heart failure (which increases the pro-arrhythmic effects of class III antiarrhythmics).5 In patients with polymorphic VT with hemodynamic compromise, first-line management is electrical cardioversion.1,10 In TdP, any offending drugs should be discontinued, electrolyte abnormalities should be corrected, and intravenous magnesium sulfate should be administered to those with long QT syndrome.1,10 In this setting, magnesium is thought to reduce the amplitude of EADs by inhibiting calcium influx through transmembrane channels, reducing the likelihood EADs reach the threshold potential necessary to trigger and sustain TdP.11 For those with recurrent TdP and bradycardia, ventricular overdrive pacing or treatment with isoproterenol (to increase heart rate and therefore shorten the QT interval) may be considered, especially for those with acquired QT interval prolongation and pause-dependent TdP, wherein sudden slowing of the heart rate precipitates TdP due to maladaptation of the QT interval to a changing heart rate.1,10,12
There are several potential explanations for why the physician did not recognize hemolysis. Hemolysis may not be flagged or readily apparent in some electronic health records (EHR).13 It is also plausible that the physician unconsciously associated hyperkalemia with the patient’s recently modified dietary intake in conjunction with poor renal clearance, as evidenced by the elevated serum creatinine level. Nonetheless, the physician should have reviewed the EHR to ascertain baseline renal function and any previous episodes of hyperkalemia, as chronic hyperkalemia in association with chronic kidney disease may not require prompt treatment.6 The combination of hyponatremia and hyperkalemia is explained by a limited number of conditions (e.g., renal failure, adrenal insufficiency, and hypoaldosteronism), and seeing both abnormalities could have presented an opportunity to formulate a differential diagnosis and repeat lab testing to confirm these findings before acting upon them.14
Approaches to Improving Safety and Patient Safety Target
With unexpected, abnormal, or incongruent clinical findings, the significance and accuracy of available data should be questioned, and instinctive or reflexive interventions should be avoided. Clinicians should have a low threshold to repeat diagnostic testing such as routine bloodwork and 12-lead ECG, particularly when the risks of testing are minimal relative to the risks of misdiagnosis and incorrect treatment. In this case, the discordance between the patient’s labs and ECG should have prompted early retesting, facilitating the recognition of pseudohyperkalemia. The case also illustrates that when approaching a patient with chest pain, clinicians should pay careful attention to the ECG, form their own assessment, and not rely on machine readings that may be inaccurate. For example, prolongation of the QT interval is not always recognized by the ECG machine, might only be recognized after careful manual scrutiny, and is an important clinical finding associated with increased risk of ventricular arrhythmia.
Furthermore, this case serves as a reminder that patients can decompensate rapidly from electrolyte abnormalities. In certain circumstances, even routine medications such as furosemide or insulin are not benign. As described above, it is important to consider the urgency with which hyperkalemia should be treated. Even when prompt treatment is required, clinicians should be cautious in giving multiple potassium-lowering medications simultaneously, and should consider monitoring with telemetry and pulse oximetry and repeat follow-up testing within an hour or two. Finally, when resuscitating patients in cardiac arrest, responders should be mindful of the side effects of medications that are routinely, and so often hastily, used. For example, sodium bicarbonate can lower serum potassium and is not recommended in the latest 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC). It should only be considered in specific situations, such as preexisting severe metabolic acidosis, hyperkalemia, or poisoning from salicylates or tricyclic antidepressants.15,16
System Optimization and Quality Improvement Approach
There are several ways in which the management and outcome in this case could have been improved, and similar events could be prevented in the future.
- Reducing incidence of hemolysis in laboratory specimens to improve data accuracy:
- Nursing and phlebotomy staff should be counseled on proper techniques to reduce in vitro hemolysis during and after sample collection.
- If hemolysis is detected, clinical laboratory workflows should exist to automatically prompt repeat testing using alternative blood samples that are not hemolyzed, if available. Alternatively, a new sample should be collected.
- In non-urgent situations, laboratory testing through the core laboratory should be encouraged over point-of-care analyzers that may be unable to detect hemolysis.3
- Increasing awareness of spurious results due to hemolysis:
- If lab samples are hemolyzed, the care team should be notified directly by lab staff via pager, text, or telephone.
- Alternatively, the clinical laboratory may consider withholding the reporting of any lab results affected by hemolysis.
- EHR systems should incorporate easily seen indicators and notifications when hemolysis is detected.
- A system of “closed loop” communication should be implemented between clinicians and lab technicians and/or the EHR interface to affirm acknowledgment of hemolyzed lab samples.
- Increasing awareness of long QT syndrome:
- Similar to alerts for patient allergies or device implants, EHRs should incorporate notifications for patients with a history of prolonged QT interval, particularly when a QT-prolonging medication has been ordered for the patient.17,18
- Improving safety of hyperkalemia treatment:
- Protocols for the treatment of hyperkalemia should be based on severity and should include indications for telemetry monitoring of patients at high risk of developing severe hypokalemia.
- Order sets for hyperkalemia should encourage (or even automate) repeating serum potassium levels shortly after treatment to assess response and guide subsequent therapy.
Take-Home Points
- Failure to recognize lab values affected by hemolysis can lead to serious patient outcomes, including death.
- Presumptive hyperkalemia should be clinically correlated with the patient’s history, physical examination, and ECG. Inconsistent findings should prompt consideration of lab error (e.g., pseudohyperkalemia).
- Clinicians should familiarize themselves with the ECG manifestations of and treatment options for severe electrolyte abnormalities, including both hypokalemia and hyperkalemia.
- Clinical data, including machine ECG interpretations and automated laboratory results, should be independently verified and correlated with the patient’s clinical presentation, especially when discordant or contradictory.
- Severe hypokalemia may be rapidly induced by aggressive treatment of hyperkalemia and can lead to life-threatening ventricular arrhythmias.
Justin L. Devera, MD
Cardiovascular Medicine Fellow
Department of Internal Medicine, Division of Cardiology
UC Davis Health
jpdevera@ucdavis.edu
David K. Barnes, MD, FACEP
Consulting Editor, AHRQ’s Patient Safety Network (PSNet)
Health Sciences Clinical Professor
Director of Faculty Development
Director of ED Sustainability
Department of Emergency Medicine
Physician Advisor
UC Davis Health
dbarnes@ucdavis.edu
William R. Lewis, MD
Professor
Department of Internal Medicine, Division of Cardiology
UC Davis Health
wrlewis@ucdavis.edu
References
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15(10):e73-e189. [Available at]
- Lippi G, von Meyer A, Cadamuro J, et al. Blood sample quality. Diagnosis (Berl). 2019;6(1):25-31. [Free full text]
- O’Hara M, Wheatley EG, Kazmierczak SC. The Impact of Undetected In Vitro Hemolysis or Sample Contamination on Patient Care and Outcomes in Point-of-Care Testing: A Retrospective Study. J Appl Lab Med. 2020;5(2):332-341. [Free full text]
- Khodorkovsky B, Cambria B, Lesser M, Hahn B. Do hemolyzed potassium specimens need to be repeated? J Emerg Med. 2014;47(3):313-317. [Free full text]
- Weiss JN, Qu Z, Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol. 2017;10(3). [Free full text]
- Kim MJ, Valerio C, Knobloch GK. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2023;107(1):59-70. [Free full text]
- Wang X, Han D, Li G. Electrocardiographic manifestations in severe hypokalemia. J Int Med Res. 2020;48(1):300060518811058. [Free full text]
- Lazzara R. Antiarrhythmic drugs and torsade de pointes. Eur Heart J. 1993;14 Suppl H:88-92. [Free full text]
- Diercks DB, Shumaik GM, Harrigan RA, et al. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2):153-160. [Free full text]
- ACC/AHA/ESC. [ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden death]. Kardiologiia. 2011;51(7):65-96. [PubMed]
- Thomas SHL, Behr ER. Pharmacological treatment of acquired QT prolongation and torsades de pointes. Br J Clin Pharmacol. 2016;81(3):420-427. [Free full text]
- Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144(10):823-839. [Free full text]
- Krasowski MD. Educational case: hemolysis and lipemia interference with laboratory testing. Academic Pathology. 2019;6:2374289519888754. [Free full text]
- Part 10.1: Life-Threatening Electrolyte Abnormalities. Circulation. 2005;112(24_suppl):IV-121-IV-125. [Free full text]
- Perman SM, Elmer J, Maciel CB, et al. 2023 American Heart Association Focused Update on Adult Advanced Cardiovascular Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2024;149(5):e254-e273. [Free full text]
- Panchal AR, Bartos JA, Cabañas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S366-S468. [Free full text]
- Sorita A, Bos JM, Morlan BW, et al. Impact of clinical decision support preventing the use of QT-prolonging medications for patients at risk for torsade de pointes. J Am Med Inform Assoc. 2015;22(e1):e21-7. [Free full text]
- Tisdale JE, Jaynes HA, Kingery JR, et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014;7(3):381-390. [Free full text]



