Don't Dismiss the Dangerous: Obstetric Hemorrhage
Main EK. Don't Dismiss the Dangerous: Obstetric Hemorrhage. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2016.
Main EK. Don't Dismiss the Dangerous: Obstetric Hemorrhage. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2016.
Case Objectives
- List the common causes of obstetric hemorrhage and the need for a unit-standard diagnosis and treatment algorithm.
- Describe the national Safety Bundle for Obstetric Hemorrhage.
- Identify obstetric hemorrhage when it is concealed or obscured by other obstetric diagnoses such as preeclampsia.
- Recognize the twin issues of "denial and delay" and understand how they lead to poor outcomes for obstetric hemorrhage.
The Case
A 19-year-old woman, pregnant at 35 weeks gestation, was admitted to the labor and delivery unit in the setting of new hypertension. Blood tests revealed a hemoglobin level of 11.1 g/dL and mildly elevated liver enzymes (aspartate aminotransferase [AST] 205 mg/dL and alanine aminotransferase [ALT] 189 mg/dL).
Given concerns for preeclampsia and for viability of the fetus, she was taken to the operating room for an emergency cesarean delivery. The delivery was uncomplicated with an expected degree of bleeding; the infant was delivered in good condition. The mother was taken to the recovery room in stable condition. A postoperative hemoglobin level was 9.6 g/dL.
Over the next few hours, the patient had progressive tachycardia and persistent hypertension. Out of concern for pulmonary embolism, a computed tomography scan was ordered, which showed no evidence of pulmonary embolism. She continued to have worsening tachycardia (heart rate in the 140s) and some new abdominal discomfort.
Repeat blood tests were done 8 hours after the previous ones and showed a hemoglobin level of 3.6 g/dL. Her AST was 1185 mg/dL and her ALT 1344 mg/dL. She was given blood transfusions and taken back to the operating room out of concern for postpartum hemorrhage. In the operating room, approximately 3 liters of blood were evacuated from the uterus. A source of bleeding could not be found so the uterus was packed with gauze, and she was transferred to the intensive care unit in critical condition.
Over the next few hours, the patient had progressive shock, respiratory failure from acute respiratory distress syndrome, and disseminated intravascular coagulation. She required vasopressors, mechanical ventilation, and more than 10 units of packed red blood cells. She had a long and complicated hospital course but was ultimately discharged to a rehabilitation facility with no major permanent injuries.
The Commentary
by Elliott K. Main, MD
Obstetric hemorrhage is the most common serious complication of childbirth. It is the major cause of maternal mortality in under-resourced settings, remains a frequent cause of preventable maternal deaths in the United States, and is the leading cause of severe maternal morbidity in all high resource countries.(1-3) Furthermore, the rate of obstetric hemorrhage appears to be increasing in the US, United Kingdom, and Australia.(4-5) For these reasons, major efforts are now underway by the American Congress of Obstetricians and Gynecologists (ACOG) and many state and national quality collaboratives to craft a more organized and structured approach to prevention and early treatment of hemorrhage.(6-8)
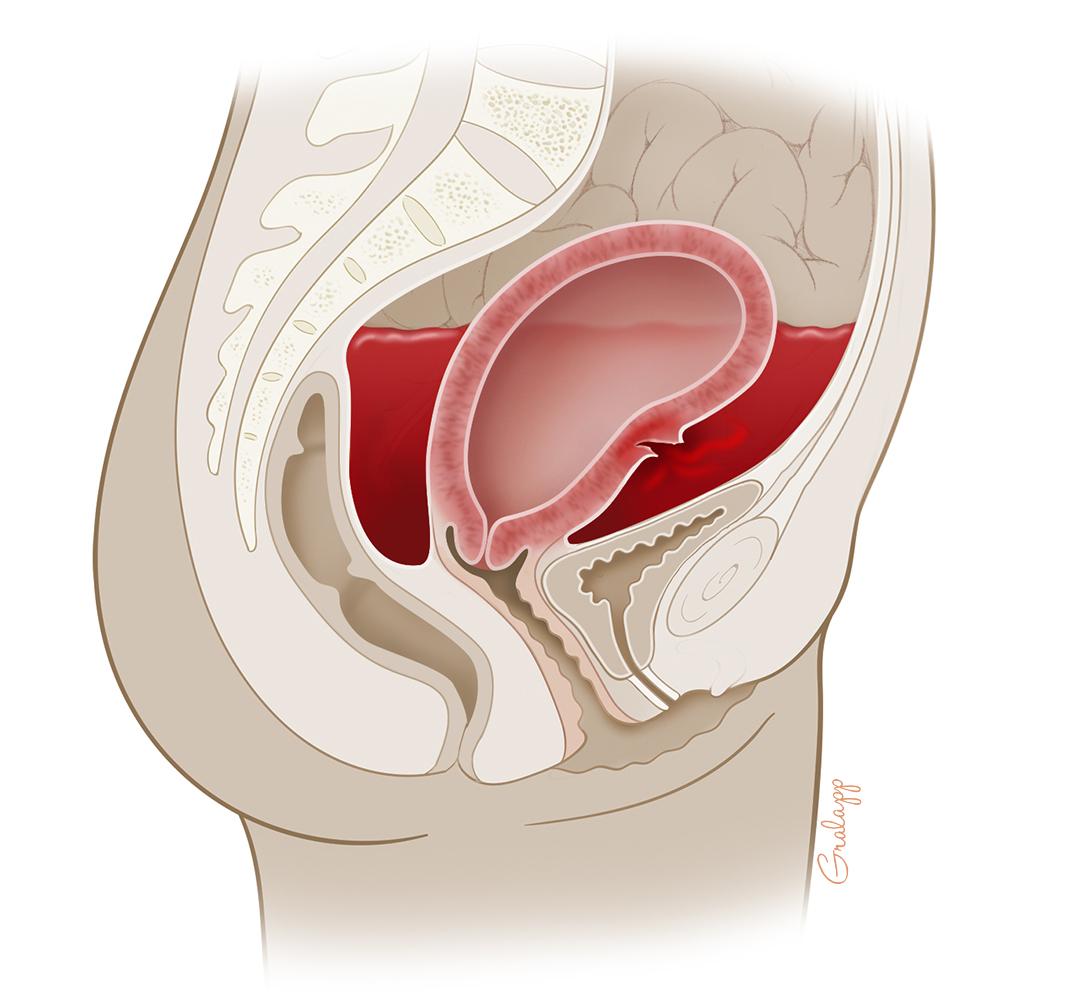
Concealed hemorrhage (internal bleeding without outward appearance of blood loss) creates additional challenges for diagnosis and treatment (Figure). Concealed hemorrhage in the context of additional pregnancy diagnoses that mask some of the key signs of hemorrhage is the most difficult of all. Examples of confounding diagnoses include preeclampsia and other hypertensive disorders of pregnancy as well as underlying cardiac disorders that feature tachycardia. This case allows us to examine routine and concealed obstetric hemorrhage as well as those obscured by confounding diagnoses and provide clinical strategies to avoid unnecessary delays.
One of the difficulties with obstetric hemorrhage is the lack of a standard, universally accepted definition. The definition most widely used in the US has been blood loss at vaginal delivery that exceeds 500 mL or at cesarean delivery that exceeds 1000 mL. There are many problems with this definition. First, there never has been a good explanation as to why the type of delivery should affect the definition, and thus defining by different volumes of blood may be inappropriate. Second, these volumes do not take into account careful research suggesting that the blood loss at delivery is frequently considerably greater than the oft-recorded 350 mL for vaginal birth and 600 mL for cesarean delivery.(9) Third, this definition does not recognize that the measurement of actual blood loss is often inaccurate. A recent ACOG-convened consensus panel tried to address these shortcomings and chose to define obstetric hemorrhage as: "Cumulative blood loss of ≥1000ml or blood loss accompanied by sign/symptoms of hypovolemia within 24 hours following the birth process."(10) The authors stress several elements: the estimation of blood loss should be as quantitative as possible rather than the usual glance and guess, it should be cumulative (bleeding can continue after the provider has left the delivery room), and maternal vital signs can be an important independent marker for significant hemorrhage.
Before we discuss the modern approach to treating obstetric hemorrhage, let us discuss the special challenges of recognizing concealed hemorrhage as illustrated in this case. In general, the diagnosis is based on signs and symptoms of hypovolemia in the absence of visible blood loss. Concealed hemorrhage most commonly occurs during a ruptured ectopic pregnancy or following a cesarean delivery with bleeding from a loosened uterine artery suture, an unrecognized laceration that occurred during the procedure, or in the setting of a coagulopathy. Concealed bleeding can also occur after a vaginal birth, typically from a cervical laceration that extends beyond the vagina into the peritoneal cavity or a vaginal wall injury with arterial bleeding creating a large sidewall hematoma. It is common for providers to miss or ignore signs of obstetric hemorrhage leading to a slow response (sometimes referred to as denial and delay). This is particularly an issue for concealed hemorrhages.(6) In such cases, hypotension is explained away as "the result of spinal or epidural anesthesia," or we just have to wait a bit longer for it to "wear off." A persistent tachycardia is "because of pain." Women complaining of increased postoperative abdominal pain are just "overly sensitive." As a surgeon, I understand the subconscious impulse to deny that you may have just caused a major operative complication and the reluctance to reopen the abdomen. But rather than denial, there are concrete steps to take.
In the recovery room or the immediate postoperative period, hypotension or tachycardia (which may actually precede hypotension) that does not respond immediately to a fluid bolus needs careful bedside evaluation for concealed hemorrhage. While not commonly appreciated, intraperitoneal bleeding can stimulate a vagal response, which can in turn blunt the expected tachycardia. This phenomenon has been well documented in healthy young women with ruptured ectopic pregnancies and can also be seen following post-cesarean bleeding.(11) Although laboratory tests should be sent, in the setting of an acute hemorrhage, a hematocrit or hemoglobin may not fully reflect the blood loss. Often, a bedside sonogram will identify a significant intra-abdominal fluid collection. But the most important step is to keep an open mind and to evaluate for concealed hemorrhage as other diagnoses are considered.
This patient was even more complicated because of the additional diagnosis of severe preeclampsia—new onset hypertension and laboratory values consistent with HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets). Preeclampsia is a form of vasospastic hypertension that can mask the usual hypotensive response to hypovolemia. In this case of concealed hemorrhage, there was no initial hypotension. While there was tachycardia (a response that helped maintain her circulation), this finding led her physicians to focus on other potential diagnoses such as pulmonary embolism.
To reiterate, the most important point in this case is the need to keep an open mind (i.e., continually contemplate a full differential diagnosis) and not attribute signs and symptoms to a solitary diagnosis—a cognitive error known as premature closure. Preeclampsia is a diagnosis that is particularly challenging in this regard as its signs and symptoms can vary greatly among patients adding to the complexity of the differential diagnosis. The coagulopathy typically seen with HELLP syndrome likely contributed to the bleeding in this patient. The coagulopathies with HELLP syndrome often worsen in the first 24–48 hours postdelivery, as occurred in this patient.(12) Furthermore, the operative field may appear dry during the procedure when the patient's blood pressure is low, only to have significant bleeding later emerge when severe hypertensive pressures return, after regional anesthesia has dissipated.
The second key point is that concealed hemorrhage has to be high on the list when either hypotension or tachycardia appears in the postoperative period. Either abnormal vital sign should be enough to trigger concern. You don't need both classical signs of hypovolemia to start an evaluation, especially in the setting of preeclampsia.
Management of Obstetric Hemorrhage
Depending on the definition used and quality of the reporting system, hemorrhage is estimated to complicate 2%–6% of all births.(5,13,14) Severe hemorrhage requiring blood transfusion occurs in 0.5% of all births, but 1%–2% of cesarean deliveries.(15) It accounts for well over half of all severe maternal morbidities.(16) The major causes of obstetric hemorrhage include uterine atony (failure of the uterus to contract after childbirth, which leads to bleeding as the normal contraction compresses blood vessels in the uterus), vaginal or cervical lacerations, and placental separation issues (retained placenta, placenta previa, and the various forms of placenta accrete). Of these, uterine atony accounts for 70%–80% of cases. If uterine atony is recognized as a cause of hemorrhage, oxytocin and fundal massage (direct massage of the uterus through the abdomen) can be used to try to firm-up uterine tone. If the atony is treated, the provider needs to make a quick assessment for lacerations and retained placental fragments as these also could have led to the bleeding. Good visibility is critical to make this evaluation.
If bleeding is recognized, the standard next steps include administering secondary medications like methergine and prostaglandins (misoprostol or carboprost [Hemabate]), followed as needed by mechanical devices to control bleeding, such as intrauterine balloons or compression sutures. Aggressive blood transfusions with fresh frozen plasma or other clotting factors to prevent disseminated intravascular coagulation are an important, and often delayed, step. If medications and mechanical devices are unsuccessful and bleeding continues, uterine artery embolization using interventional radiology can be employed. A series of supportive measures need to occur simultaneously (monitoring, laboratory studies, and maintaining normal body temperature and oxygenation). The final option in severe, persistent bleeding is total hysterectomy, but it is used only as a last resort.
In obstetrics, we see many cases where the bleeding will stop with uterine massage only. This leads to a presumption that it will in every case, creating a sense of denial: "The bleeding will stop soon."; "It is not that much."; "She is young and healthy." This denial, often driven by inadequate estimation of actual blood lost, can lead to delays in care that may turn an otherwise easily recoverable situation into a life-threatening event.
The seriousness of the problem and complexity of the response make it imperative for institutions to organize a standardized emergency response plan. The National Partnership for Maternal Safety operating under the Council on Patient Safety in Women's Health Care (a consortium of major obstetric, nursing, and anesthesia societies) has created a national Safety Bundle for Obstetric Hemorrhage that is highly recommended for all obstetricians to read and all maternity units to implement.(6) The Safety Bundles are comprised of four domains: readiness, recognition/prevention, response, and reporting (Box). Readiness ensures that the unit is prepared for immediate action in the case of hemorrhage. This includes having a hemorrhage cart full of the equipment needed, rapid access to key medications, a preorganized response team, protocols with the blood bank for emergency release and massive transfusions, and of course educational programs for the response. Recognition/prevention includes assessing every patient for their risk of hemorrhage to better prepare, semiquantitatively measuring cumulative blood loss to avoid the denial and delay so commonly seen, and universally using oxytocin following delivery. Response has two components: develop a unit-standard, stage-based response protocol with checklists and deploy resources on the unit for supporting patient families and staff in the event of a major hemorrhage, or other significant obstetric emergency. Tested examples of all of these tools are available.(6,8) Reporting and systems learning actions, including huddles, debriefs, and monitoring outcomes, are the glue that creates the culture of safety on the unit and maintains the ability to respond.
The first step to implement such a program is to create a team that includes key obstetricians, nurses, anesthesiologists, pharmacists, administrators, and representatives from the blood bank. Many states now have perinatal quality collaboratives that can provide assistance. The Council on Patient Safety in Women's Health Care has great resources available at http://www.safehealthcareforeverywoman.org, and the California Maternal Quality Care Collaborative (8) has a comprehensive toolkit at https://www.cmqcc.org.
In conclusion, obstetric hemorrhages are common and remain a major cause of severe maternal morbidity and even preventable death. They also serve as a valuable rallying point to create a culture of safety on maternity units by implementing the national safety bundle. Especially in the setting of preeclampsia, concealed hemorrhage can stretch our diagnostic skills, but having the back-up of a team working from a standard plan can make all the difference.
Take-Home Points
- Responding to an obstetric hemorrhage requires a plan and a well-practiced team. Especially for emergency situations, standardized evaluation and response is strongly recommended.
- "Denial and delay" are common in the evaluation of obstetric hemorrhage.
- Concealed hemorrhage has to be high on the list when either hypotension or tachycardia appears in the postoperative period.
- Until a cause or a diagnosis is fully determined, it is important to keep an open mind (i.e., continually contemplate a full set of differential diagnoses) and not focus on a specific diagnosis.
- Every obstetric provider should carefully study the national Council on Patient Safety in Women's Health Care's Consensus Bundle on Obstetric Hemorrhage and work to implement it in their maternity unit.
Elliott K. Main, MD Medical Director California Maternal Quality Care Collaborative
Faculty Disclosure: Dr. Main has declared that neither he, nor any immediate member of his family, has a financial arrangement or other relationship with the manufacturers of any commercial products discussed in this continuing medical education activity. In addition, the commentary does not include information regarding investigational or off-label use of pharmaceutical products or medical devices.
References
1. Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstet Gynecol. 2005;106:1228-1234. [go to PubMed]
2. Main EK, McCain CL, Morton CH, Holtby S, Lawton ES. Pregnancy-related mortality in California: causes, characteristics, and improvement opportunities. Obstet Gynecol. 2015;125:938-947. [go to PubMed]
3. Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036. [go to PubMed]
4. Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. [go to PubMed]
5. Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States 1994–2006. Am J Obstet Gynecol. 2010;202:353.e1-353.e6. [go to PubMed]
6. Main EK, Goffman D, Scavone BM, et al; Council for Patient Safety in Women's Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162. [go to PubMed]
7. Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212:272-280. [go to PubMed]
8. Lyndon A, Lagrew D, Shields L, Main E, Cape V, eds. Improving Health Care Response to Obstetric Hemorrhage, Version 2.0: A California Toolkit to Transform Maternity Care. Stanford, CA: California Department of Public Health, Maternal, Child and Adolescent Health Division, California Maternal Quality Care Collaborative; March 2015. [Available at]
9. Rath WH. Postpartum hemorrhage—update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand. 2011;90:421-428. [go to PubMed]
10. Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153. [go to PubMed]
11. Adams SL, Greene JS. Absence of a tachycardic response to intraperitoneal hemorrhage. J Emerg Med. 1986;4:383-389. [go to PubMed]
12. Martin JN Jr, Blake PG, Perry KG Jr, McCaul JF, Hess LW, Martin RW. The natural history of HELLP syndrome: patterns of disease progression and regression. Am J Obstet Gynecol. 1991;164:1500-1509. [go to PubMed]
13. Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999-1012. [go to PubMed]
14. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047. [go to PubMed]
15. Klapholz H. Blood transfusion in contemporary obstetric practice. Obstet Gynecol. 1990;75:940-943. [go to PubMed]
16. Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol. 2016;214:643.e1-643.e10. [go to PubMed]
Box
Box. Obstetric Hemorrhage Safety Bundle From The Council on Patient Safety in Women's Health Care (ACOG, AWHONN, ACNM, SMFM, SOAP, and Others)
| Readiness (Every Unit) |
|---|
|
| Recognition and Prevention (Every Patient) |
|
| Response (Every Hemorrhage) |
|
| Reporting and Systems Learning (Every Unit) |
|
Figure
Figure. Illustration of Concealed Hemorrhage, Showing a Laceration of the Uterine Wall With Bleeding Into the Peritoneal Cavity. (Illustration © 2016 Chris Gralapp.)