Do Not Miss Sepsis Needles in Viral Haystacks!
Hamline M, Shaikh U. Do Not Miss Sepsis Needles in Viral Haystacks!. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Hamline M, Shaikh U. Do Not Miss Sepsis Needles in Viral Haystacks!. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Patrick Romano, MD, MPH; Debra Bakerjian, PhD, APRN, RN; Michelle Hamline, MD, PhD, MAS; and Ulfat Shaikh, MD, MPH for this Spotlight Case and Commentary have disclosed no relevant financial relationships with ineligible companies related to this CME activity.
Learning Objectives
At the conclusion of this educational activity, participants should be able to:
- Differentiate between pediatric sepsis and septic shock.
- Explain the importance of timely identification and appropriate management of pediatric sepsis.
- Identify tools to assist the clinician in recognizing sepsis in the pediatric patient.
- Recognize common cognitive biases that may contribute to diagnostic errors in missing sepsis.
The Case
A five-year-old fully immunized girl was brought to the emergency department (ED) for an upper respiratory infection with symptoms of fever, cough, runny nose, nausea, sore throat, dyspnea, generalized weakness, and rash. There was no vomiting or diarrhea. On examination, vital signs were as follows: temperature 38.3 Celsius, pulse 143 beats per minute (bpm), respiratory rate 24 per minute, and oxygen saturation 100%. There were normal breath sounds and a maculopapular rash. A viral swab was negative for SARS-CoV2, influenza, and respiratory syncytial virus. The treating physician did not mention pneumonia in the differential diagnosis.
On a visit the next day, there was more generalized weakness and new upper abdominal pain. Vital signs were as follows: temperature 39.5, pulse markedly tachycardic at 205 bpm, respiratory rate markedly tachypneic at 36 per minute, and oxygen saturation 94%. Physical examination revealed normal breath sounds, enlarged tonsils, and a “sandpapery” rash without petechiae. Laboratory testing showed a normal white blood cell count of 8,500, but with 1.6% abnormal metamyelocytes and burr cells. The serum sodium was low at 127 mEq/L, and the bicarbonate was also low at 16 mEq/L, with a normal anion gap of 12. Blood urea nitrogen (BUN) and creatinine were normal. A throat swab was positive for group A Streptococcus. The treating physician again did not order imaging and attributed all findings to pharyngitis. The child was sent home with a prescription for amoxicillin.
On day 3 after the first ED visit, the child was brought back to the ED by ambulance with pulseless electrical activity at a heart rate of 70 bpm and oxygen saturation of 40% with no spontaneous respirations. On examination during resuscitation, there was skin mottling and petechiae. She was pronounced dead after resuscitative efforts failed. Autopsy showed bilateral pneumonia and right-sided empyema. Empyema cultures grew Streptococcus pyogenes and Klebsiella pneumoniae.
The Commentary
By Michelle Hamline, MD, PhD, MAS, and Ulfat Shaikh, MD, MPH
Background
This tragic case describes a failure to identify sepsis in a pediatric patient, leading to the development of septic shock and ultimately death over the following 48 hours. Autopsy demonstrated bilateral pneumonia with right-sided empyema. Despite two emergency department (ED) visits in the days preceding her rapid clinical decline, the patient’s sepsis was not diagnosed, and it appears that pneumonia was not explicitly considered in the differential diagnosis. A throat swab was positive for group A streptococcus, a presumptive diagnosis of viral upper respiratory infection was made, and a chest x-ray was not performed. This patient’s experience emphasizes the critical importance of timely recognition and proper management of potential bacterial infections to prevent downstream morbidity and mortality from sepsis.
Patient Safety Targets
Recognition of Pediatric Sepsis
Infections have been implicated in 25% of childhood deaths worldwide.1 Sepsis, which broadly refers to the body’s overwhelming, non-specific inflammatory response to infection, remains a significant cause of mortality in pediatric patients. While sepsis has long been recognized in the medical literature, both its definition and the criteria used to diagnose it have transformed over time.2
A 2005 international consensus conference developed the initial criteria for the diagnosis of sepsis in pediatric patients, characterizing sepsis as suspected or confirmed infection in the setting of an overwhelming systemic inflammatory response syndrome (SIRS).3 A diagnosis of SIRS relied on the presence of at least two age-based abnormalities among the following parameters: temperature, heart rate, respiratory rate (or requirement for mechanical ventilation), and leukocyte count. Additional criteria primarily relying on the diagnosis of organ dysfunction further defined severe sepsis and septic shock. While these definitions have been widely adopted, their limitations include poor specificity, lack of reliability in identifying children at risk of poor outcomes and concern that clinicians may be misled into thinking that sepsis follows a clear continuum from SIRS to sepsis, followed by severe sepsis and ultimately septic shock.
In adults, these concerns led to the new Sepsis-3 consensus definitions for adult sepsis and septic shock, published in 2016.4 The new definition redefined sepsis more narrowly as a life-threatening acute organ dysfunction due to a dysregulated host response to infection. Its diagnosis incorporates the use of the Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score to represent degree of organ dysfunction, with a score of 2 points or higher associated with in-hospital mortality over 10%.
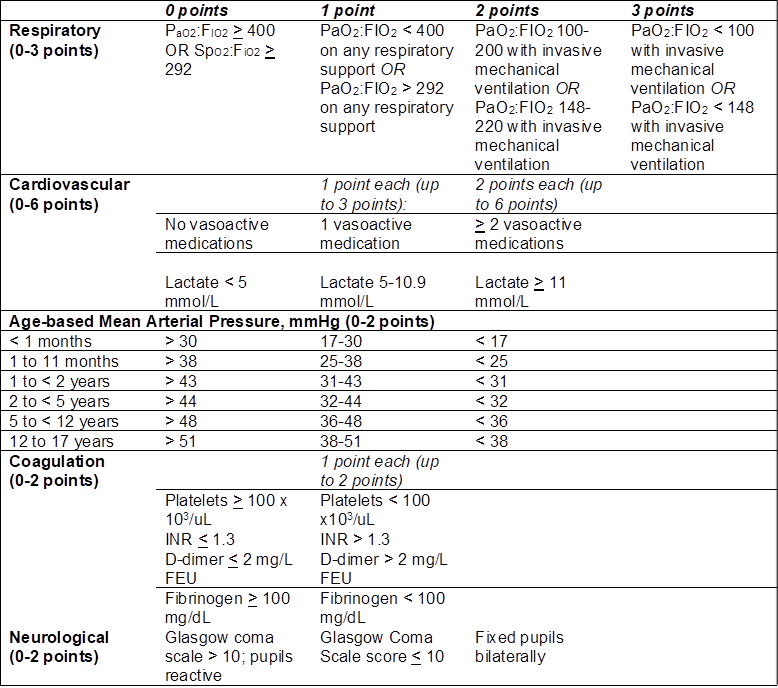
However, recognizing that sepsis in children is distinct from adult sepsis, particularly in its sepsis-related comorbidities, epidemiology, and outcomes, the pediatric community has long been working to develop an updated definition of sepsis specific to children,5-9 which was published in January 2024.10,11 Similar to the adult Sepsis-3 criteria, the Phoenix Sepsis Score, shown in Table 1, eliminates the prior SIRS-based criteria, reconceptualizing sepsis as “life-threatening acute organ dysfunction secondary to a dysregulated host response to infection.” The score relies on evaluation of 4 organ systems—respiratory, cardiovascular, coagulation, and neurological—with sepsis defined as a cumulative score of 2 or greater in the setting of suspected infection. This recharacterization of pediatric sepsis has improved correlation with mortality risk; patients diagnosed with sepsis by these criteria have in-hospital mortality of 7.1% in higher resource settings and 28.5% in lower resource settings. Septic shock, which is defined as a diagnosis of sepsis with at least 1 cardiovascular point, is associated with even greater mortality risk—10.8% in higher resource and 33.5% in lower resource settings.
Table 1. Phoenix Sepsis Score
Source: table adapted from Schlapbach, et al. 2024.
Fibrinogen equivalent units = FEU; International Normalized Ratio = INR
It is unclear whether the patient in this case met the updated Phoenix criteria for a diagnosis of sepsis in her initial ED visits, given the concern for potential infection. She was initially febrile to 38.3o Celsius with tachycardia at 143 bpm and tachypnea at 24 breaths per minute. At five years of age, two of these values exceed age-appropriate cut-offs used in the 2005 criteria for diagnosis of pediatric SIRS. If the suspected etiology is viral, as in the patient’s initial presentation, the typical practice is to administer an antipyretic (if febrile) and intravenous or oral hydration (if concerned for dehydration) to determine whether vital signs normalize with the resolution of fever and dehydration. If vital signs fail to normalize with these measures, the patient would typically be observed or admitted for further monitoring. In addition, further evaluation of blood pressure and laboratory test results against the Phoenix Sepsis Score criteria could have allowed identification of developing sepsis and early intervention, which may have mitigated the negative outcomes that subsequently developed.
Ultimately, the goal of early sepsis recognition is the rapid implementation of appropriate treatment. In 2020, the Surviving Sepsis Campaign published guidelines for management of suspected sepsis and septic shock in children.12 These guidelines recommend expedited diagnostic evaluation for any child in whom sepsis is suspected, followed by rapid intravenous or intraosseous access, blood cultures, empiric antibiotic therapy, and administration of fluid boluses within one hour of the diagnosis of septic shock. While guidelines for managing sepsis in adult patients reflect the benefits of initiating volume resuscitation and empiric antibiotic therapy as quickly as possible, less evidence exists to guide the management of pediatric sepsis without septic shock. While the patient described in this case does not appear to have met criteria for septic shock on her second ED presentation, she should have received expedited diagnostic evaluation given her highly abnormal vital signs.
Identifying Pneumonia and Its Potential Complications
Pneumonia remains a common cause for hospitalization of children in the United States. During the above patient’s second ED visit, the patient’s vital signs suggested the potential for worsening illness, with fever to 39.5o Celsius, tachycardia to 205 bpm, and tachypnea to 36 breaths per minute. Furthermore, her oxygen saturation had declined to 94%. These findings prompted additional laboratory evaluation, which further revealed hyponatremia, a non-anion gap metabolic acidosis, and metamyelocytes and burr cells on her complete blood count. Particularly in the setting of a new complaint of upper abdominal pain, these findings should have raised the suspicion for pneumonia, even in the absence of focal lung findings on exam. While a non-anion gap metabolic acidosis and immature cell types may be non-specific in the setting of infection, hyponatremia is known to be associated with community-acquired pneumonia, with the degree of hyponatremia thought to be correlated with pneumonia severity.13,14,15 Recognition of this association, as well as tachypnea and decreased oxygen saturation, should have prompted the team to obtain a chest radiograph and additional biomarkers, such as lactate, C-reactive protein, and pro-calcitonin.
On autopsy, the patient was found to have not only bilateral Streptococcus pyogenes and Klebsiella pneumonia, but also right-sided empyema. While Streptococcus pyogenes has been implicated rarely in community-acquired pneumonia, Klebsiella pneumoniae is among the top five bacterial pathogens causing pneumonia and has been implicated in some particularly severe cases.16 Empyema complicates 3% of all pneumonia hospitalizations.17 Notably, the incidence of pediatric empyema rose nearly 70% in the years following introduction of the pneumococcal conjugate vaccine in 2000, even while bacterial pneumonia and other invasive pneumococcal disease declined in incidence.18 In addition to empyema, other potential complications of pneumonia include necrosis, abscess, and parapneumonic effusion. Such complications are often not suspected based on the physical examination alone; a high index of suspicion to obtain imaging is needed (i.e., x-ray, followed by ultrasound or computed tomography if needed). Such imaging may have aided in the diagnosis of the child described above, as earlier treatment of her empyema may have prevented her demise.
Diagnostic Errors
Diagnostic errors are common, estimated at around 5.7% in ED settings, with some estimates ranging up to 10 to 15% in certain fields.19 Cognitive biases are often caused by inappropriate mental models or limitations in human processing and are a frequent contributor to diagnostic errors.20 Common types of cognitive biases that play a role in diagnostic errors include:
- Confirmation bias, which refers to selective information-gathering with subsequent interpretation reflecting current beliefs. Often, evidence that contradicts these prior beliefs is neglected, which can lead to misdiagnosis. This can also lead to diagnostic momentum, in which incorrect diagnoses are passed on to subsequent providers who accept them without questioning their validity.21
- Anchoring, which is similar to confirmation bias but focuses on clinicians’ prioritization of information and data that support their first impressions. This may contribute to premature closure, in which clinicians may prematurely settle on a particular diagnosis, based on initial information, to the exclusion of other more likely diagnoses, as occurred in the described case.21
- Implicit bias, which refers to bias in how we perceive other individuals, including patients, according to observable characteristics such as race, ethnicity, socioeconomic status, or even age. Implicit biases may contribute to an affect heuristic, in which a clinician’s actions are influenced by emotion, rather than rational deliberation.22 While we have limited knowledge of the patient’s demographic characteristics in this case, we must consider the possibility that her age and sex influenced clinician decision-making toward a less aggressive diagnostic approach.
Attention to the issue of cognitive bias should be balanced by an acknowledgement of the role of intuition and professional judgment. The Naturalistic Decision Making (NDM) framework23,24 considers the benefits of professional experience, sensemaking, pattern recognition, and situational awareness in decision-making. In addition to the deliberate analysis of a complex scenario using clinical decision support tools, NDM recognizes that clinicians frequently need to utilize their intuition and experience to rapidly make decisions in health care environments that are complex, uncertain, and rapidly changing.
In health care, we are frequently taught that “common diagnoses are most common.” Viral respiratory infections are common in pediatrics. They often present like the child described in this case, which may have led clinicians to anchor on this diagnosis. The patient’s positive rapid throat swab and diagnosis of streptococcal pharyngitis may have further contributed to premature closure. During the patient’s second presentation to the ED, several red flags for sepsis and a more serious bacterial infection such as pneumonia (e.g., worsening tachycardia, tachypnea, and oxygen saturation; hyponatremia) were apparently not given adequate consideration, possibly due to cognitive biases, challenges with recalling or interpreting age-based vital signs and laboratory results, or environmental distractions or interruptions.
Approaches to Improving Safety
Improved Detection of Pediatric Sepsis
A potential intervention that can help clinicians interpret vital signs is the use of easily accessed printed or electronic resources containing age-specific pediatric vital sign ranges. In addition to web resources, a variety of applications are now available for clinicians to download to their mobile devices.
A more sophisticated approach, if available, involves incorporating age-specific pediatric vital sign ranges into the electronic health record (EHR) and generating automated alerts for patients with abnormal vital signs. Such alerts avoid reliance solely on a clinician’s memory for determining appropriate age-specific pediatric vital signs and may help identify patients with risk for sepsis in a timelier manner by leveraging interruptive alerts. EHR-based sepsis alerts may improve the identification of sepsis and help reduce the time to initiation of appropriate treatment.25,26 While such alerts may have relatively low specificity for identifying patients with sepsis, they tend to have high sensitivity and may be paired with a clinician’s judgment to rule out those patients for whom infection is unlikely.25,26 Even more advanced forms of EHR-based clinical decision support that incorporate artificial intelligence and machine learning may further increase diagnostic accuracy of sepsis,25,27,28 but these tools require external validation as their performance in different settings may be disappointing.23,24
Clinical Decision Support Tools for the Identification of Bacterial Pneumonia
Another intervention to mitigate cognitive biases is a clinical decision tool to guide additional diagnostic workup. Recent efforts have sought to identify the risk of radiographic pneumonia in pediatric patients based on a variety of clinical findings and biomarkers. In 2022, Lipsett and colleagues published a Pneumonia Risk Score (PRS) to predict the risk of radiographic pneumonia in children 3 months to 18 years of age based on the patient’s age, initial oxygen saturation, presence of fever, rales, and wheeze.29 By assessing the likelihood of radiographic pneumonia, the score helps clinicians to determine which patients should receive a chest x-ray. With a fever and no crackles or wheezing on exam, the patient described above would have had a PRS of 3 on initial presentation, rising to a 4 on her second ED visit, indicating a moderate risk of pneumonia that would prompt the clinician to consider obtaining a chest x-ray.
Other clinical decision support tools have proposed the use of various biomarkers to help distinguish bacterial pneumonia from viral lower respiratory tract infection. One of the more promising proposed biomarkers is procalcitonin, which has been established as associated with community-acquired pneumonia in adults. However, its role in children has not been as well-established. One recent study showed that elevated procalcitonin (> 0.5 ng/mL) demonstrates a sensitivity of 29.7% and specificity of 87.5% in identifying radiographic pneumonia in children.30 Further, procalcitonin levels tend to be higher in children with more severe pneumonia.31
Improving Diagnostic Accuracy
Although educating clinicians about cognitive biases, their potential contribution to diagnostic errors, and methods of avoiding them may help to improve clinical decision-making,12 guided reflection in searching for and identifying potential alternative diagnoses may be a more effective strategy.32,33 This strategy typically employs a supportive mentor to challenge diagnoses and help the clinician engage in thoughtful reasoning. Cognitive forcing strategies, which implement conscious consideration of alternative diagnoses, may also have some efficacy.33,34 These methods may employ use of standardized workflows to encourage clinicians to continue their diagnostic search, avoiding premature closure. These strategies require additional research to assess their feasibility in fast-paced clinical environments and their effectiveness in preventing diagnostic errors and improving diagnostic timeliness.
Take-Home Points
- Sepsis, which refers to the body’s overwhelming, non-specific inflammatory response to infection, remains a significant cause of mortality in pediatric patients. Age-specific vital signs and laboratory values are used to diagnose sepsis in children and adolescents.
- The goal of early sepsis recognition is the rapid implementation of appropriate treatment. Guidelines recommend expedited diagnostic evaluation for children for whom sepsis is suspected, followed by rapid intravenous or intraosseous access, blood culture, initiation of empiric antibiotic therapy, and administration of fluid boluses in children within one hour of diagnosis of septic shock.
- Viral respiratory infections are common in pediatrics and may be challenging to distinguish from early signs of more serious infections. Diagnostic errors, cognitive biases, and challenges with recalling or interpreting age-based vital signs and laboratory results may lead to missing red flags for sepsis and serious bacterial infections.
- Clinical decision support tools for detection of sepsis such as alerts and predictive risk scores integrated into electronic health record systems can aid clinicians in interpreting age-specific pediatric vital sign ranges and prompt appropriate diagnostic studies, especially in fast-paced and high-acuity health care settings.
Michelle Hamline, MD, PhD, MAS
Assistant Professor
Associate Director of Quality and Safety
Department of Pediatrics
UC Davis Health
mhamline@ucdavis.edu
Ulfat Shaikh, MD, MPH
Associate Editor, AHRQ’s Patient Safety Network (PSNet)
Professor
Department of Pediatrics
Medical Director for Healthcare Quality
UC Davis Health
ushaikh@ucdavis.edu
References
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. [Free full text]
- Carlton EF, Perry-Eaddy MA, Prescott HC. Context and implications of the new pediatric sepsis criteria. JAMA. 2024;331(8):646-649. [Free full text]
- Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2-8. [Free full text]
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-810. [Free full text]
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):775-787. [Free full text]
- Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171(10):e172352. [Free full text]
- Morin L, Hall M, de Souza D, et al. The current and future state of pediatric sepsis definitions: an international survey. Pediatrics. 2022;149(6):e2021052565. [Free full text]
- Carrol ED, Ranjit S, Menon K, et al. Operationalizing appropriate sepsis definitions in children worldwide: considerations for the Pediatric Sepsis Definition Taskforce. Pediatr Crit Care Med. 2023;24(6):e263-e271. [Free full text]
- Menon K, Schlapbach LJ, Akech S, et al. Criteria for pediatric sepsis-a systematic review and meta-analysis by the Pediatric Sepsis Definition Taskforce. Crit Care Med. 2022;50(1):21-36. [Free full text]
- Schlapbach LJ, Watson RS, Sorce LR, et al. International consensus criteria for pediatric sepsis and septic shock. JAMA. 2024;331(8):665-674. [Free full text]
- Sanchez-Pinto LN, Bennett TD, DeWitt PE, et al. Development and validation of the Phoenix Criteria for Pediatric Sepsis and Septic Shock. JAMA. 2024;331(8):675-686. [Free full text]
- Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46(suppl 1):10-67. [Free full text]
- Nair V, Niederman MS, Masani N, et al. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27(2):184-190. [Available at]
- Don M, Valerio G, Korppi M, et al. Hyponatremia in pediatric community-acquired pneumonia. Pediatr Nephrol. 2008;23(12):2247-2253. [Available at]
- Królicka AL, Kruczkowska A, Krajewska M, et al. Hyponatremia in infectious diseases-a literature review. Int J Environ Res Public Health. 2020;17(15):5320. [Free full text]
- Rudan I, Boschi-Pinto C, Biloglav Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86(5):408-416. [Free full text]
- Clark JE, Hammal D, Spencer D, et al. Children with pneumonia: how do they present and how are they managed? Arch Dis Child. 2007;92(5):394-398. [Free full text]
- Li ST, Tancredi DJ. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics. 2016 125(1):26–33. [Available at]
- Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(5 suppl):S2-23. [Free full text]
- Croskerry P. From mindless to mindful practice--cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448. [Available at]
- Doherty TS, Carroll AE. Believing in overcoming cognitive biases. AMA J Ethics. 2020;22(9):e773-e778. [Free full text]
- Balakrishnan K, Arjmand EM. The impact of cognitive and implicit bias on patient safety and quality. Otolaryngol Clin North Am. 2019;52(1):35-46. [Available at]
- Kahneman D, Klein G. Conditions for intuitive expertise: a failure to disagree. Am Psychol. 2009;64(6):515-526. [Available at]
- Klein DE, Woods DD, Klein G, et al. Can we trust best practices? Six cognitive challenges of evidence-based approaches. J Cogn Eng Decis Mak. 2016;10(3):244-254. [Available at]
- Wulff A, Montag S, Marschollek M, et al. Clinical decision-support systems for detection of systemic inflammatory response syndrome, sepsis, and septic shock in critically ill patients: a systematic review. Methods Inf Med. 2019;58(S 02):e43-e57. [Available at]
- Ackermann K, Baker J, Green M, et al. Computerized clinical decision support systems for the early detection of sepsis among adult inpatients: scoping review. J Med Internet Res. 2022;24(2):e31083. [Free full text]
- Petersen C, Smith J, Freimuth RR, et al. Recommendations for the safe, effective use of adaptive CDS in the US healthcare system: an AMIA position paper. J Am Med Inform Assoc. 2021;28(4):677-684. [Free full text]
- Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. [Free full text]
- Lipsett SC, Hirsch AW, Monuteaux MC, et al. Development of the novel pneumonia risk score to predict radiographic pneumonia in children. Pediatr Infect Dis J. 2022;41(1):24-30. [Free full text]
- Ratageri VH, Panigatti P, Mukherjee A, et al. Role of procalcitonin in diagnosis of community acquired pneumonia in children. BMC Pediatr. 2022;22(1):217. [Free full text]
- Sartori LF, Zhu Y, Grijalva CG, et al. Pneumonia severity in children: utility of procalcitonin in risk stratification. Hosp Pediatr. 2021;11(3):215-222. [Free full text]
- Mamede S, Schmidt HG. The structure of reflective practice in medicine. Med Educ. 2004;38(12):1302-1308. [Free full text]
- Lambe KA, O'Reilly G, Kelly BD, et al. Dual-process cognitive interventions to enhance diagnostic reasoning: a systematic review. BMJ Qual Saf. 2016;25(10):808-820. [Free full text]
- Croskerry P. Cognitive forcing strategies in clinical decisionmaking. Ann Emerg Med. 2003;41(1):110-120. [Available at]