Benefits vs. Risks of Intraosseous Vascular Access
Fowler RL, Lippmann MJ. Benefits vs. Risks of Intraosseous Vascular Access. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2014.
Fowler RL, Lippmann MJ. Benefits vs. Risks of Intraosseous Vascular Access. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2014.
The Case
A 72-year-old woman with a history of asthma, congestive heart failure, and medication noncompliance presented to the emergency department with 2 weeks of lower extremity edema, fatigue, and progressively worsening dyspnea. She reported shortness of breath at rest and with exertion, as well as a dry cough. On initial examination, she was wheezing and had notable right lower extremity erythema and bilateral lower extremity pitting edema greater on the right side with weeping from her skin. She was admitted for asthma exacerbation and lower extremity cellulitis. She improved with fluids, albuterol nebulizers, methylprednisolone, and ceftriaxone/doxycycline. During her next 2 hospital days, she had a lower extremity ultrasound that was negative for a deep vein thrombosis and a transthoracic echocardiogram that was normal except for biatrial enlargement.
At midnight of her second hospital day, the patient's son noted that his mother was feeling dizzy. Four hours later, the patient suddenly became bradycardic to a heart rate of 20 beats per minute. Walking to the bathroom, she was notably dyspneic, with an oxygen saturation of 87%. She then became unresponsive. Her initial rhythm was pulseless electrical activity. During the code, a senior resident placed an intraosseous (IO) line in the left tibia following several unsuccessful attempts to obtain peripheral venous access. After 10 minutes of chest compressions and advanced cardiovascular life support protocol, spontaneous circulation returned and the patient was transferred to the intensive care unit (ICU).
Three hours after the IO line was placed, a nurse notified the primary team that the left leg was a dusky purple, and on examination the leg was bluish and tensely edematous with sluggish distal pulses. Vascular surgery diagnosed compartment syndrome, removed the IO line, and performed a bedside fasciotomy later that morning. The fasciotomy wounds were slow to heal and required ongoing complex care. After 2 months in the ICU and multiple complications, the patient was discharged.
The Commentary
Vascular access is a cornerstone of modern medical therapeutics. In a crisis situation, particularly one in which large volumes of fluids or vasoactive medications are required, physicians have been trained to access the vascular system through large veins in the neck or groin. Yet, particularly when the blood pressure or volume is low, or in children, intravenous (IV) access can be difficult, and many patients have died over the years because of inability to achieve access in a timely way.
Placing a vascular access device directly into the marrow cavity for medical treatment dates as far back as the 1920s.(1) These devices were used during World War II for the treatment of shock (2), but later fell out of favor in many countries until their reintroduction in the United States to pediatric resuscitations in the late 1980s. Their resurgence in adult medicine lagged and only regained popularity in the past decade.(3) The modern development of rapid and easy-to-use intraosseous (IO) placement devices has recently placed IO access within the grasp of the broad realm of advanced life support providers.(4)
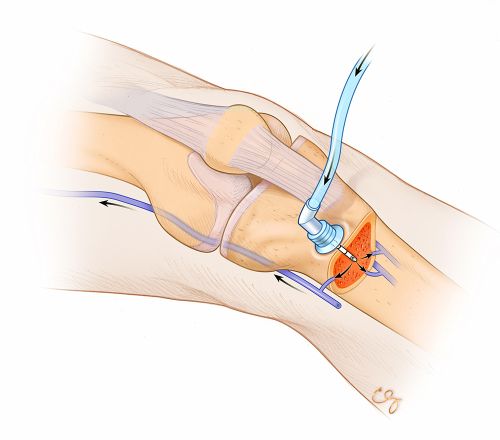
The technique of IO line placement is now a well-accepted component of modern advanced life support algorithms.(3) Intraosseous access requires either drilling or punching through the bone cortex and placing a hollow needle in the marrow cavity, within which is a rich network of marrow vasculature that will rapidly transport fluids and medications into the vascular system at large (Figure). Generally, the penetrated bony cortex holds the IO device firmly, making the line more difficult to accidentally displace (though displacements may occur).
After establishing a favorable safety and efficacy profile, IO access is endorsed as a preferred first alternative to failed IV access by numerous professional bodies including the American Heart Association (AHA), American College of Emergency Physicians (ACEP), International Liaison Committee on Resuscitation (ILCOR), and National Association of EMS Physicians (NAEMSP). Intraosseous access placement by nonphysician providers is commonly employed and is proven to be time-saving in emergency resuscitation cases that frequently arise in both the prehospital and emergency department settings.(3,5)
The operator should use reasonable guidelines in employing IO access. Infected sites should be avoided in seeking access. Generally, if IO access has already been used and abandoned on one extremity, the opposite extremity should be used for future attempts due to the potential for leakage from the original site if IO access is placed close to it. Manufacturers' guidelines for choosing access device size should be followed. Patients with excessive tissue may require a larger-sized needle to gain IO access and decrease the likelihood of displacement of the IO device.
The flow dynamics through IO access are more complex than when the access is directly into a vein. The rate of parenteral fluid flow of the access device can vary depending on needle size, access site, and dynamics of the marrow space (including the possibility of bone spicules obstructing flow out of the device). For example, in the setting of low flow into a tibial space, the operator may find that rotating the device a few degrees may improve flow. Users should be cautious about aspirating blood and marrow back through the device, such as for the purpose of obtaining laboratory studies, as this aspiration may cause the access needle to become obstructed. The flow of fluid through the humeral head is superior—up to five times higher—to that in the tibial space, approaching the flow into a central access line (L. Miller, oral communication, October 2013).
Numerous complications may arise with the use of IO devices.(6) As in the present case, the access needle's point of penetration into the cortex may leak, allowing infusion of fluid and medications into the surrounding tissues, creating local extravasation, and possibly creating a compartment syndrome. A Scandinavian survey of 1800 cases in which an IO device was used for vascular access revealed complications such as patient discomfort and pain (7.1%), difficulties penetrating the periosteum with the IO needle (10.3%), trouble with aspiration of bone marrow (12.3%), and bent or broken IO needles (4.0%). Other reported problems included difficulty with injection of fluid and drugs after IO insertion (7.4%), slow infusion (8.8%), displacement after insertion (8.5%), and extravasation (3.7%). Of note, compartment syndrome, as occurred in this case, was relatively rare—10 cases (0.6%). There were also 6 cases (0.4%) of osteomyelitis.(6) Meticulous attention to the site surrounding the IO catheter is essential to monitor for complications, such as extravasation in this patient; her compartment syndrome likely could have been prevented if the extravasation had been discovered earlier. Lastly, once the urgency of the situation has resolved, replacing the IO needle with more long-term IV access is advised and will further decrease complications associated with IO access.
Even though IO access devices have a low complication rate, they are substantially more costly (often exceeding $100) than IV catheters (a few dollars). These costs can become substantial for emergency response systems. For example, in the 12 months following the initial rollout of the IO device in the Dallas area (7), the emergency medical services (EMS) system had to absorb an increase in costs in excess of $40,000. Therefore, it is reasonable for EMS medical directors to establish a protocol requiring some number of initial peripheral IV attempts prior to implementing IO use.
Standardized initial and continuing training should be in place, consistent with medical director requirements and manufacturers' recommendations. ACEP advocates developing institutional policies and training for the use of IO access.(8) Also, manufacturers of the devices have straightforward training materials available to guide the administrative training staff of hospitals and EMS agencies that employ these devices.
In our experience, most cases of IO access go well. However, when there are problems, it is usually because of poor choice of IO insertion site, IO access attempted in the presence of known contraindications, or incorrect placement of IO needle (either too deep [through back wall of bone in infants] or too shallow [attempted use of insufficiently long needle in adults with obesity]). These complications can be mitigated by developing detailed institutional policy guidelines for IO use, and by rigorously training all individuals who may place IO needles in proper insertion technique.
Take-Home Points
- Intraosseous vascular access may be lifesaving, and emergency response systems should have one of these devices readily available with personnel trained in their use.
- The complication rate associated with IO use is small and comparable to IV access.
- IO vascular access is more expensive than IV catheters, and the risks and benefits of various access devices should be weighed when deciding which to use.
Raymond L. Fowler, MD Professor of Emergency Medicine, Surgery, Health Professions, and Emergency Medical Education University of Texas Southwestern Medical Center Dallas, TX
Melanie J. Lippmann, MD Assistant Professor of Emergency Medicine Warren Alpert Medical School Brown University Providence, RI
References
1. Drinker CK, Drinker KR, Lund CC. The circulation in the mammalian bone-marrow. Am J Physiol. 1922;62:1-92.
2. Morrison GM. The initial care of casualties. Am Pract Dig Treat. 1946;1:183. [go to PubMed]
3. Phillips L, Brown L, Campbell T, Miller J, Proehl J, Youngberg B. Recommendations for the use of intraosseous vascular access for emergent and nonemergent situations in various health care settings: a consensus paper. Crit Care Nurse. 2010;30:e1-e7. [go to PubMed]
4. Luck RP, Haines C, Mull CC. Intraosseous access. J Emerg Med. 2010;39:468-475. [go to PubMed]
5. Vidacare Corp. ACEP policy statement endorses use of intraosseous vascular access as alternative to intravenous vascular access in the ED. JEMS. November 8, 2011. [Available at]
6. Hallas P, Brabrand M, Folkestad L. Complication with intraosseous access: Scandinavian users' experience. West J Emerg Med. 2013;14:440-443. [go to PubMed]
7. Fowler RL. The electronic crossroads: opportunities and challenges in medical data management. JEMS. October 1, 2009. [Available at]
8. American College of Emergency Physicians. Alternative methods to vascular access in the emergency department. Ann Emerg Med. 2011;58:402. [go to PubMed]
Figure
Figure. Left leg with intraosseous device placed in proximal tibia. (Illustration © 2014 Chris Gralapp.)