Under Pressure: Delayed Diagnosis of Compartment Syndrome after Lower Leg Fracture.
Barnes DK, Randhawa SDS, Fitzpatrick EP. Under Pressure: Delayed Diagnosis of Compartment Syndrome after Lower Leg Fracture.. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Barnes DK, Randhawa SDS, Fitzpatrick EP. Under Pressure: Delayed Diagnosis of Compartment Syndrome after Lower Leg Fracture.. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
The Cases
Case #1: A 24-year-old man was seen in a trauma center after his motorcycle collided with a car. Imaging showed a left tibial plateau fracture. He was admitted to an orthopedic unit for anticipated surgery. Within hours, he complained of “excruciating” pain and numbness in his toes in his injured leg. He was seen by an intern and senior resident overnight, both of whom documented concern that the patient could be developing compartment syndrome. However, they were reassured by palpable dorsalis pedis pulses and subsidence of the pain, so the possibility of compartment syndrome was not investigated further. Over the next 12 hours, the patient’s leg pain worsened, and the leg became quite swollen. The following day, he was taken to the operating room for open reduction with internal fixation of his tibial fracture. His left calf was noted to be very tense; the surgeon confirmed elevated compartment pressures and performed fasciotomies for compartment syndrome. Nevertheless, the patient later required re-operation to excise necrotic muscle tissue leading to a chronic deformity and impaired strength in the affected leg.
Case #2: A 19-year-old college student suffered a severe left tibiofibular fracture when he was side-tackled while playing indoor soccer. The patient was taken by ambulance to the nearest hospital and underwent emergency surgery (open reduction with internal fixation) by an on-call orthopedic surgeon. Throughout the night and into the following morning, the patient experienced severe pain, numbness, a burning sensation, and reduced muscle strength in his lower left leg. The pain was difficult to control even with opioid medications. The patient was discharged that afternoon despite continuing to report extreme pain. He returned to the hospital six days later when his symptoms did not improve. The patient underwent a second operation performed by a different surgeon who identified acute compartment syndrome. The muscles in the anterior compartment of the patient’s leg were gray, had no contractility, and required extensive debridement. He underwent multiple subsequent operations to restore function but was left with some permanent disability and disfigurement.
The Commentary
By David K. Barnes, MD, FACEP, Sahej Deep Singh Randhawa, MD, and Ellen P. Fitzpatrick, MD
Although diagnosing acute compartment syndrome (CS) can be difficult, both patients in these cases displayed typical symptoms of CS involving lower extremity fractures: development of severe worsening pain and neurologic deficits (i.e., numbness and weakness). In the first case, the treating team did not recognize preoperative CS despite the patient’s complaint of severe pain and neurologic symptoms in the injured extremity. The resolution of pain was misleading and insufficient to exclude CS. Although the on-call team considered CS in this patient with a high-energy mechanism and higher-risk fracture type, they were falsely reassured by the presence of palpable distal pulses. CS can lead to a pulse deficit, but this is typically a late finding. Moreover, the presence of pulses does not rule out CS. The trainees should have escalated their concerns to a more experienced clinician who likely would have pursued the diagnosis of CS more aggressively.
In the second case, the treating team failed to appreciate symptoms suggesting postoperative CS and instead discharged the patient home despite his complaint of worsening, severe pain unresponsive to typical opioid doses. This symptom was a red flag that should have alerted them to stop the discharge and determine the cause of his pain. Pain out of proportion to injury severity is a hallmark sign of CS and should have been investigated prior to discharge. It is not clear why it took 6 days for the patient to return for evaluation; the diagnosis likely would have been made sooner if the patient had received clear instructions to return if his pain continued, or if he had received close post-discharge follow-up via text, telephone, or an in-person visit. This patient may have had a similar outcome even if CS had been diagnosed earlier after discharge, but the initial failure to suspect and investigate for CS was the primary cause of the poor outcome. Both cases represent failures of timely diagnosis and intervention; permanent loss of muscle tissue and functional impairment could have been prevented with appropriate suspicion, surveillance, and diagnostic testing.
Background
Acute compartment syndrome (CS) is a limb-threatening emergency caused by elevated pressure within a myofascial compartment severe enough to impair circulation. While CS can be caused by burns, vascular occlusion, and iatrogenic or idiopathic processes, it is mostly attributed to trauma from fractures or crush injuries. The most common location of CS is the lower leg, but other compartments are also at risk, including the hand, foot, buttock, forearm, and thigh.1–3
Regardless of the etiology or anatomic location, the underlying process is similar: an initial trigger (e.g., fracture, crush, or reperfusion injury after a period of ischemia) causes muscle swelling; blood and tissue fluids then accumulate within the inelastic fascial compartment; the pressure within that compartment rises leading to venous hypertension; and a self-perpetuating cycle of increased pressure ensues. Eventually, compartment pressure exceeds diastolic blood pressure, arresting perfusion and causing ischemia. Cellular anoxia and tissue necrosis follow if intervention is delayed. Timely fasciotomy relieves the intracompartmental pressure and restores perfusion to the affected tissues.2,4
Paradoxically, late-presenting patients who have sustained this aforementioned process of CS may report improved or resolved pain. Because of the spectrum of severity of CS, resolved pain may indicate that an opportunity to intervene still exists, or that CS has progressed such that ischemia is irreversible and muscle tissue is no longer viable. Unfortunately, clinicians may incorrectly interpret pain resolution as a positive development and abort further diagnostic evaluation.3 Delayed diagnosis occurs more commonly in patients who have difficulty perceiving pain in the injured extremity (e.g., after regional or spinal anesthesia) or reporting their symptoms (e.g., patients with altered mental status, pre-verbal children).1
An extensive review of the pathophysiology of CS can be found in a previous PSNet commentary related to intraosseous line extravasation causing CS in a pediatric patient.
Diagnosis
Historically, CS has been diagnosed by bedside assessment using various clinical signs and symptoms. Pain out of proportion to exam, increasing analgesic requirements, pain with passive stretch of the affected muscles, sensory changes (e.g., numbness, paresthesia), motor changes (e.g., weakness, paralysis), and swelling are associated with CS. The “6 Ps” of limb ischemia have also been associated with CS and refer to pain, pallor, pulselessness, paresthesias, paralysis, and poikilothermia (an inability to maintain core body temperature).
It is customary to assess for the presence or absence of distal pulses when evaluating for CS, but CS can be neither ruled in if pulses are absent nor ruled out if pulses are present. Pulselessness, pallor, and paralysis are late findings of CS and can be misleading when used as diagnostic tools for CS. Unless there is an associated arterial injury, compartment pressure is rarely elevated sufficiently to compromise arterial pressure and cause pulselessness. By similar mechanism, pallor is uncommon and present only in the setting of severely diminished arterial inflow.4,5
Compromised neurologic function is common in CS. Sensory changes are typically the first sign of nerve ischemia. Paresthesias are an early sign of CS that can progress to hypesthesia and anesthesia if perfusion is not restored. Motor impairment, including paralysis, is typically a late finding.5
Compartment fullness or firmness can be a manifestation of increased pressure and an early sign of CS; it can be assessed regardless of a patient’s mental status or ability to sense pain. However, a study using a cadaveric model of CS demonstrated that palpation of compartment firmness is an insensitive and unreliable finding, with a sensitivity of 24% and specificity of 55% for correct identification of elevated pressure in the affected compartment.6 Severe pain out of proportion to the patient’s injury and pain that does not improve with appropriate analgesia are also concerning signs of CS, although they may be confounded by opioid treatment. Pain with passive stretch of the muscle within the affected compartment is the most sensitive exam finding.7
Although compartment syndrome is considered a clinical diagnosis, evidence has shown that even positive clinical findings do not reliably predict CS. A systematic review evaluating the commonly accepted clinical signs of CS (i.e., pain, pain on passive stretch, paresthesias, and paresis) reported low sensitivity but high specificity, giving them poor predictive value in many clinical settings.8 The American Academy of Orthopaedic Surgeons’ (AAOS) “Appropriate Use Criteria (AUC) Summary” on the diagnosis and management of compartment syndrome noted that no physical examination finding or diagnostic test has 100% specificity or sensitivity for CS, and diagnostic criteria should vary based on patient factors (e.g., altered mental status).9 For example, the previously outlined clinical features may not be applicable to children who may be unable to articulate symptoms. Instead, children may show signs of agitation, anxiety, and increasing analgesic need (i.e., the three As).10
A manometer can be used to measure the pressure within an affected compartment yielding objective data that may support the clinical assessment and confirm clinical suspicion for CS. Manometry is most often used to check ICP intermittently, but continuous ICP manometers are also available. McQueen et al. reported sensitivities ranging from 13 to 64% for clinical findings compared with 94% for compartment pressure monitoring using an intracompartmental pressure (ICP)-to-diastolic blood pressure difference (∆P) of less than 30 mmHg for more than 2 hours. Specificities for clinical findings are better yet only range from 63 to 98% compared with 98% for compartment pressure monitoring. Not surprisingly, these authors recommend that “peripheral pulses, pallor, and diminished capillary return should not be used to determine a diagnosis” of CS.11
It is tempting to think ICP measurement alone would be superior to the insensitive history and physical exam. However, ICP monitoring is subject to measurement error and has been associated with unacceptably high rates of false positives and false negatives, especially if measurements are not obtained at an ideal anatomic location or proper technique is not utilized. In a cadaveric study using a handheld manometer, 30% of users performed catastrophic technique errors, and of those tests performed correctly, 40% of measurements deviated more than 5 mmHg above the actual pressure.12 Using ICP measurement may therefore unnecessarily increase the frequency of fasciotomy. In one study of 19 patients with lower extremity fractures and no clinical evidence of CS, 16 patients (84%) had at least one ∆P measured at less than 30 mm Hg, the most common value of ICP used to diagnose CS. None of these patients developed CS or required surgery for CS, but they would have undergone fasciotomy unnecessarily if ICP alone was used to determine the need for intervention.13 Thus, experts recommend that ICP supplement the clinical assessment and be used to confirm clinical suspicion of CS rather than as an isolated diagnostic tool.1
Despite everything summarized above, consensus is lacking regarding the pressure threshold causing muscle ischemia, the duration of muscle ischemia tolerated, indications for and timing of fasciotomy, and the utility of any given clinical finding. For example, although a ∆P less than 30 mmHg is the most recommended pressure threshold for diagnosing ischemia and thus CS, some authors advocate the use of an absolute ICP greater than 30 mmHg. The AAOS Clinical Practice Guideline Summary for the Management of Acute Compartment Syndrome provides only limited to moderate strength recommendations, noting “the dearth of high-quality research precluded the group from making strong recommendations regarding diagnosis and treatment.”9
Approaches to Improving Patient Safety
Diagnostic Evaluation of Compartment Syndrome
Diagnosing CS can be challenging because many common pre- and postoperative findings, such as pain and swelling, are also present in CS. For example, pain and swelling after operative management of a tibial shaft fracture can represent a normal postoperative course, but in the right context, they may herald development of CS. Unfortunately, none of the tools used to assess for CS—history, physical exam, or manometry—is 100% specific. There will always be some diagnostic uncertainty leading to false negatives and false positives. While delaying intervention for a probable case of CS is undesirable, unnecessary fasciotomy is associated with its own risks that some patients would find unacceptable. One author summarized this dilemma facing surgeons who are considering operating for CS:
“Thus, clinicians facing patients at risk of [acute compartment syndrome] must choose a treatment plan from among several bad choices: perform fasciotomy and expose the patient to the risks and costs associated with that procedure, or not do fasciotomy and expose the patient to the potential adverse effects of delayed fasciotomy or missed [acute compartment syndrome].”2
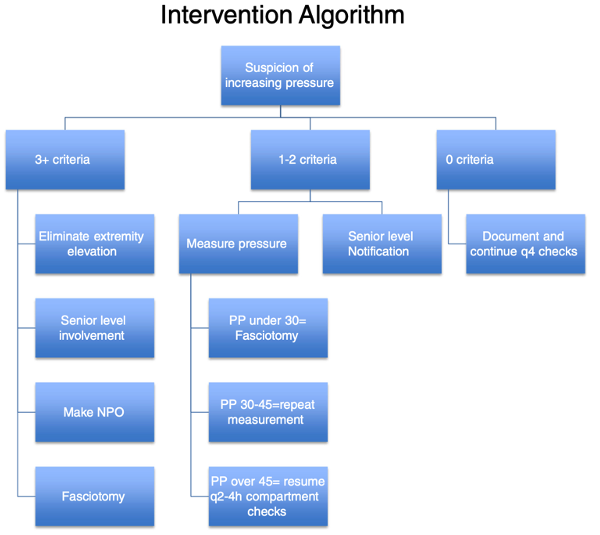
Although the ultimate decision rests with the patient, most experts agree that the consequences of delaying a necessary fasciotomy outweigh the risks of performing an unnecessary fasciotomy. Effective CS screening tools should therefore maximize sensitivity to reduce the false negative rate and thus minimize patient harm due to delayed or missed diagnosis of CS. Combining subjective patient complaints with a focused clinical examination and ICP measurement improves diagnostic sensitivity. McQueen et al. described the use of an algorithmic approach (seen here) to screen patients for CS using a combination of patent risk factors, clinical symptoms, exam findings, and ICP monitoring.11
Lack of clearly defined diagnostic criteria is a common reason for delayed diagnosis of clinical syndromes such as CS. Using a structured approach adds valuable objectivity to clinical decision making that would otherwise be subjective and prone to heuristics.
Trainees and Patient Safety
Diagnostic uncertainty was not the only factor that contributed to the errors in these cases. In the second case, obvious, classic symptoms of CS were ignored, and ICP was never measured. However, in the first case, the involvement of trainees raises additional questions unrelated to the clinical assessment:
- Were the trainees appropriately supervised?
- Did they escalate their concerns about CS to an appropriate supervisor (e.g., attending physician)?
- Did resident duty hours or shift work play a role in the delayed diagnosis, and if so, how?
Residency training is a critical period for a physician to develop professionally. Skills acquisition and conditional autonomy must be balanced against the need to keep patients safe from harm while trainees hone their clinical and decision-making acumen. Although the training and experience of the resident team are unknown, their failure to escalate their concerns to a supervising physician suggests they lacked knowledge or were overconfident in their abilities. Whenever trainees are involved, safeguards should exist to ensure that insufficient knowledge, skill, or ability doesn’t lead to patient harm. Concerns about life- and limb-threatening conditions like CS should be escalated to a physician with the necessary experience and authority to determine if intervention is warranted.
Following implementation of the Accreditation Council for Graduate Medical Education (ACGME) resident work hour rules in 2004 (and again in 2011 and 2017), the frequency of patient handoffs increased because resident duty periods became shorter to allow more rest.1 Conditions like CS that are dynamic and evolve over time may therefore go undetected when continuity of care is interrupted, such as during a patient handoff.
Patient handoff is one of the most common communication challenges in healthcare. Communication failure is one of the most frequently cited root causes of sentinel events.14 Despite widespread use of tools to facilitate safe handoffs, approximately half of communication failures are associated with patient handoffs.15 Healthcare teams should therefore adopt practices that consider handoff frequency and team structure, including the roles of advanced practice providers (APPs).
When multiple team members share responsibility for the care of a patient, they should communicate key findings when patient status changes and at defined time points (e.g., patient handoff between shifts, patient transferring services). Garner et al. recommend using a structured approach when patients at risk for CS are transitioned between teams:
- Identify patients at risk for CS and communicate concern with all team members.
- On-call residents should perform and document compartment checks every 2 to 4 hours and update the team’s list or handoff documentation with time performed as serial examination at different time points provides valuable information.
- Include these three main components in checks for CS:
- Assess the patient’s pain.
- Review interim analgesic requirements.
- Perform a targeted physical exam including:
- Palpation for muscle fullness
- Passive stretch of each muscle group (to elicit pain)
- Motor and sensation testing (to elicit any new abnormalities)
- Pulse and capillary refill (to elicit any new abnormalities)
- Assess the number of criteria the patient has for CS using a structured algorithm (see Figure 1). Any one of the four positive physical exam findings described above is classified as one criterion.1
- Based on the results of this algorithm, the examiner can either continue to perform serial checks (0 criteria), notify a senior physician and measure ICP (1-2 criteria), or initiate a pathway towards fasciotomy (3+ criteria).1
Figure 1. Structured Algorithm to Evaluate for CS
Source: Garner MR, et al (2014)
Note: “PP” in this Figure refers to pulse pressure, also known as “∆P” as referenced above.
This approach is useful not just for trainees but for all team members (i.e., physicians, nurses, APPs) who care for patients at risk for CS. While this approach still requires an examiner to have an initial clinical suspicion for CS, the structure of the algorithm provides discrete thresholds for escalation of diagnostic testing and intervention. In addition, we recommend concurrent bedside patient examination of patients who are being evaluated for CS when team members transition to calibrate findings across examiners.
Discharge Instructions and Post-discharge Follow-up
Hospital discharge represents a vulnerable period in a patient’s healthcare journey. Almost 1 in 5 patients experience an adverse event within the first month after hospital discharge, and many of these are preventable.16 Case 2 is representative of a preventable hospital “bounce-back” because the patient should not have been discharged. The team failed to appreciate findings suggestive of CS, especially in a high-risk context after a severe tibial fracture.
The topic of unplanned hospital readmissions has been extensively studied. (See PSNet primer: Readmissions and Adverse Events After Discharge.) Many patients are readmitted because their treatment was incomplete, because of inadequate coordination of services after discharge, or because of a problem with a medication.17 Given various economic and social pressures favoring early discharge, some patients are discharged prematurely, which tends to leave them unprepared for what to expect or do after discharge. Simple and inexpensive processes exist that may identify patients who were discharged prematurely and redirect them back to the hospital while the opportunity for effective treatment still exists.
Clear and effective discharge instructions are critical to ensure patients understand directions for self-care after leaving the hospital, and why and when they should seek medical care. Several discharge instruction tools are available to assist clinicians, such as the IDEAL Discharge Planning Tool developed by AHRQ:
- Include the patient and family as full partners in the discharge planning process.
- Discuss with the patient and family key areas to prevent problems at home.
- Educate the patient and family in plain language about the patient’s condition, the discharge process, and next steps throughout the hospital stay.
- Assess how well doctors and nurses explain the diagnosis, condition, and next steps in the patient’s care to the patient and family and use teach back.
- Listen to and honor the patient’s and family’s goals, preferences, observations, and concerns.
Contacting patients soon after discharge by text or telephone is another intervention that may alert healthcare teams to a patient who needs to return for additional care. In a study of 30,727 members of a commercial health plan, patients discharged after hospitalization who received a follow-up telephone call within 14 days of discharge were 23% less likely to be readmitted (p = 0.043).18 Our hospital implemented an automated post-discharge follow-up telephone process for discharged surgical patients, and has expanded the program to contact patients who are treated and discharged from the ED, and those who are triaged but leave before completing their treatment. Patients respond to automated questions read by a computer-generated voice. Questions are designed to identify patients who need additional coaching about their discharge instructions and those who should return to the hospital or clinic for reevaluation. Anyone responding affirmatively to a question receives a telephone call from a registered nurse trained in triage. Had a system like this been in place, the patient in Case 2 would probably have been identified earlier than 6 days after discharge and advised to return to the hospital for immediate reevaluation. The Re-Engineered Discharge (RED) Toolkit from AHRQ includes instructions “How to Conduct a Post discharge Follow-up Phone Call” that may help healthcare systems develop a post-discharge follow-up process.
Take-Home Points
- In patients at risk for compartment syndrome, maintain a high index of suspicion and perform serial assessments.
- If compartment syndrome is suspected, clinical assessment alone is unreliable. Use a combination of history, exam, analgesic requirements, and compartment pressure monitoring to improve diagnostic accuracy.
- Frequent communication between team members about dynamic clinical conditions reduces the risk of medical error during high-risk episodes of care, such as patient handoffs.
- Concerns about limb- or life-threatening conditions should be escalated to a team member with experience and decision-making authority.
- Clear discharge instructions and post-discharge follow-up can reduce the harm associated with incomplete hospital care or complications that arise after discharge.
David K. Barnes, MD, FACEP
Consulting Editor, AHRQ, Patient Safety Network (PSNet)
Health Sciences Clinical Professor
Director of Faculty Development
Director of ED Sustainability
Department of Emergency Medicine
Physician Advisor
UC Davis Health
dbarnes@ucdavis.edu
Sahej Deep Singh Randhawa, MD
Resident Physician
Department of Orthopedics
UC Davis Health
sdrandhawa@ucdavis.edu
Ellen P. Fitzpatrick, MD
Associate Clinical Professor
Department of Orthopedics
UC Davis Health
efitzpatrick@ucdavis.edu
References
- Garner MR, Taylor SA, Gausden E, et al. Compartment syndrome: diagnosis, management, and unique concerns in the twenty-first century. HSS J. 2014;10(2):143-152. [Free full text]
- Schmidt AH. Acute compartment syndrome. Orthop Clin North Am. 2016;47(3):517-525. [Available at
- Murdock M, Murdoch MM. Compartment syndrome: a review of the literature. Clin Podiatr Med Surg. 2012;29(2):301-310. [Available at]
- Ouellette EA. Compartment syndromes in obtunded patients. Hand Clin. 1998;14(3):431-450.
- Olson SA, Glasgow RR. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13(7):436-444. [Available at]
- Shuler FD, Dietz MJ. Physicians' ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92(2):361-367. [Available at]
- Konda SR, Kester BS, Fisher N, et al. Acute compartment syndrome of the leg. J Orthop Trauma. 2017;31(Suppl 3):S17-S18. [Free full text]
- Ulmer T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder?. J Orthop Trauma. 2002;16(8):572-577. [Available at]
- Osborn PM, Schmidt AH. Diagnosis and management of acute compartment syndrome. J Am Acad Orthop Surg. 2021;29(5):183-188. [Free full text]
- Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680-688. [Available at]
- McQueen MM, Duckworth AD. The diagnosis of acute compartment syndrome: a review. Eur J Trauma Emerg Surg. 2014;40(5):521-528. [Available at]
- Large TM, Agel J, Holtzman DJ, et al. Interobserver variability in the measurement of lower leg compartment pressures. J Orthop Trauma. 2015;29(7):316-321. [Available at]
- Prayson MJ, Chen JL, Hampers D, et al. Baseline compartment pressure measurements in isolated lower extremity fractures without clinical compartment syndrome. J Trauma. 2006;60(5):1037-1040. [Available at]
- Oakbrook Terrace, IL: Joint Commission Resources. 2016. Sentinel Event Statistics Released for 2015. (Accessed April 18, 2024) [Free full text (PDF)]
- Vidyarthi AR. Triple Handoff. Agency for Healthcare Research and Quality Patient Safety Network. 2006 [Accessed April 18, 2024) [Free full text]
- Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. [Available at]
- Mwachiro DM, Baron-Lee J, Kates FR. Impact of post-discharge follow-up calls on 30-day hospital readmissions in neurosurgery. Glob J Qual Saf Healthc. 2019;2(2):46–52. [Free full text]
- Harrison PL, Hara PA, Pope JE, et al. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14(1):27-32. [Free full text]