The Risks of a Malpositioned Gastrostomy Tube and Poor Communication
Hight RA. The Risks of a Malpositioned Gastrostomy Tube and Poor Communication. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2023.
Hight RA. The Risks of a Malpositioned Gastrostomy Tube and Poor Communication. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2023.
Patrick Romano, MD, MPH; Debra Bakerjian, PhD, APRN, RN; Amy A Nichols, EdD RN CNS CHSE ANEF and Rachel Ann Hight, MD, FACS for this Spotlight Case and Commentary have disclosed no relevant financial relationships with ineligible companies related to this CME activity.
Learning Objectives
At the conclusion of this educational activity, participants should be able to:
- Describe various techniques available to place long-term gastric access devices.
- Recognize common and uncommon complications of PEG tube placement.
- Describe possible causes of diagnostic error in the interpretation of radiology studies.
- Describe vulnerabilities in communication across a spectrum of care environments.
- Suggest possible solutions to improve interprofessional and interfacility communication.
The Case
A 55-year-old woman was hospitalized after a motor vehicle crash with cardiac arrest in the field. She was found to have subarachnoid and intraventricular hemorrhages from multiple cerebral aneurysms, treated with endovascular coiling and complicated by refractory intracranial hypertension requiring decompressive hemicraniectomy on hospital day 12. She underwent percutaneous tracheostomy placement on day 24 and percutaneous endoscopic gastrostomy (PEG) tube placement on day 30. The surgeon placed the PEG tube in the intensive care unit (ICU) using the “pull technique” (i.e., via the mouth) because an operating room was not available, and her computed tomography (CT) scan showed no interposed bowel between the stomach and the anterior abdominal wall. During the procedure, the abdominal wall transilluminated as expected, and one-to-one motion of the stomach occurred with external palpation. The postoperative plain film showed the gastrostomy tube bulb in an appropriate position. The surgeon cleared the patient’s team to advance tube feeds as tolerated.
Six days after PEG placement, the patient developed “intractable emesis” of tube feeds and a gastroenterology consultant was unable to identify the etiology. Intermittent tube feed intolerance continued; a repeat CT scan on day 41, based on the gastroenterologist’s request to evaluate for small bowel obstruction but without mention of the recent PEG placement, showed the gastrostomy tube in the stomach with no evidence of obstruction. On day 43, the patient underwent cranioplasty and ventriculoperitoneal (VP) shunt placement, followed on day 51 by repeat aneurysm coiling and right carotid artery stent placement. On day 64, the acute care surgery team was consulted to convert the PEG tube to a percutaneous gastrojejunostomy (GJ) tube due to the patient’s intermittent emesis; however, they instead recommended interventional radiology (IR) perform a minimally invasive tube exchange. When the IR team evaluated the patient on day 65, they re-reviewed the CT scan from 24 days earlier and noted (for the first time) that the gastrostomy tube traversed the liver for 1.7 cm. They recommended surgical revision instead; the acute care surgical team agreed to perform open revision with possible GJ tube placement after coordinating with the neurosurgery team about VP shunt management. On day 67, a repeat CT scan with gastric contrast confirmed that the transhepatic course of the tube was unchanged compared with the earlier CT scan.
The neurosurgeon recommended continuing dual antiplatelet therapy for 2 months after carotid stent placement, so the two teams opted to keep the gastrostomy tube for gastric venting and to place a nasojejunal (NJ) feeding tube. The NJ tube was placed successfully with confirmation of jejunal positioning on day 71. By the next day, the patient was tolerating goal tube feeds via the NJ tube and had weaned off parenteral nutrition. On day 73, the discharge planner left a note recommending surgery clinic follow-up in about 3 weeks to coordinate open gastrostomy tube revision after discontinuation of antiplatelet therapy. The patient was discharged to a rehabilitation facility on day 74 with orders that included a surgery follow-up appointment to “discuss GT revision to JT placement” but without clear documentation that the PEG tube was malpositioned through the liver. Around 10 days after hospital discharge, the patient was seen by the neurosurgeon, who now recommended a 3-month course of dual antiplatelet therapy with repeat cerebral angiography before stopping ticagrelor. The patient’s spouse then called the surgery clinic to change the follow-up visit; he revealed that his wife was taking enough orally that enteral access was no longer required for nutrition. Unaware that the PEG tube was malpositioned through the liver, the surgeon attending clinic that day (who had not seen the patient previously) changed the plan to gastrostomy tube removal in clinic 3-4 weeks after discontinuation of antiplatelet therapy.
Following this plan, the patient was seen in follow-up by a different surgeon (who had also not seen the patient previously) for outpatient gastrostomy tube removal. The patient had been eating full meals, but she had aphasia and her husband did not know that the PEG tube was malpositioned. The surgeon removed the tube uneventfully via the abdominal wall tract using the common traction technique. A few hours later, the clinic surgeon further reviewed the prior hospital record and noted the transhepatic course of the PEG tube. When he contacted the operating surgeon, he learned that the plan was to revise the PEG tube via laparoscopic or open technique due to concern for liver bleeding that might occur during removal. Surprised by this information, the clinic surgeon immediately contacted the patient’s husband, who reported that his wife appeared well and that her vital signs were normal. An emergent abdominal CT showed no evidence of intra-abdominal hemorrhage. On further review, it became apparent that a third surgeon had assessed the patient near the end of her inpatient stay and recommended outpatient open gastrostomy tube revision, but neither the operating surgeon nor the clinic surgeon was aware of this plan.
The Commentary
By Rachel Ann Hight, MD, FACS
Although several opportunities for improvement were identified in the review of this complex case, the primary issues were (1) the original complication of a malpositioned transhepatic PEG tube, which was a delayed diagnosis after tube feedings were poorly tolerated and the CT scan was misread, and (2) the subsequent lack of consistent communication identifying this complication and the associated plan for management of the malpositioned PEG tube. The latter problem was compounded by the need for multidisciplinary care coordination with evolving care plans for management of the dual antiplatelet therapy across transitions from the acute care hospital through inpatient rehabilitation care into outpatient care. Communication through these care hand-offs about the feeding tube reflected only “PEG tube” or “G tube” and did not specifically identify this feeding tube being “malpositioned” and/or “transhepatic”; thus, subsequent members of the care team had no awareness that the PEG tube was transhepatic.
Background Information and Significance
Percutaneous endoscopic gastrostomy (PEG) tubes are commonly utilized for long-term enteral feeding access for many different reasons,1 with about 200,000-250,000 procedures performed annually in the United States and a success rate of 95% or higher.2-6 Decisions about the type of enteral access and the means to establish enteral access are driven by patient factors such as anatomy and specific pathology and comorbidities, in the context of available facility teams and resources for placement.3,4,5
Enteral feeding access options broadly include gastrostomy tubes and jejunostomy tubes. These tubes can be placed nasally, orally, or directly through the abdominal wall. Generally, gastrostomy tubes are preferred for long-term feeding access when it is clinically appropriate because there are usually less complications related to gastrostomy tube placement and long-term management compared with jejunostomy tube placement.3,4,5,7,8 Feeding tubes placed nasally or orally can be optimized to terminate in the stomach, duodenum, or jejunum, with appropriate positioning confirmed radiographically using fluoroscopic guidance. Although these tubes are easy to place at the bedside with minimal resources (i.e., without conscious sedation), their disadvantage is that they are usually intended for 6 weeks or less, and many long-term care facilities require patients to have more durable enteral access solutions.7,8
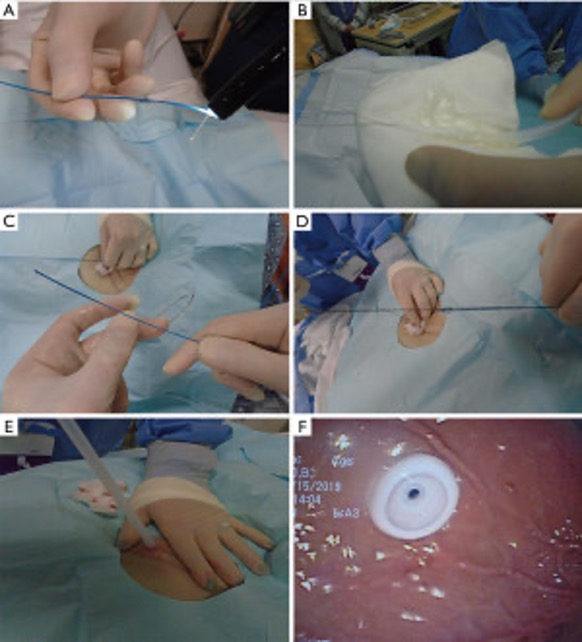
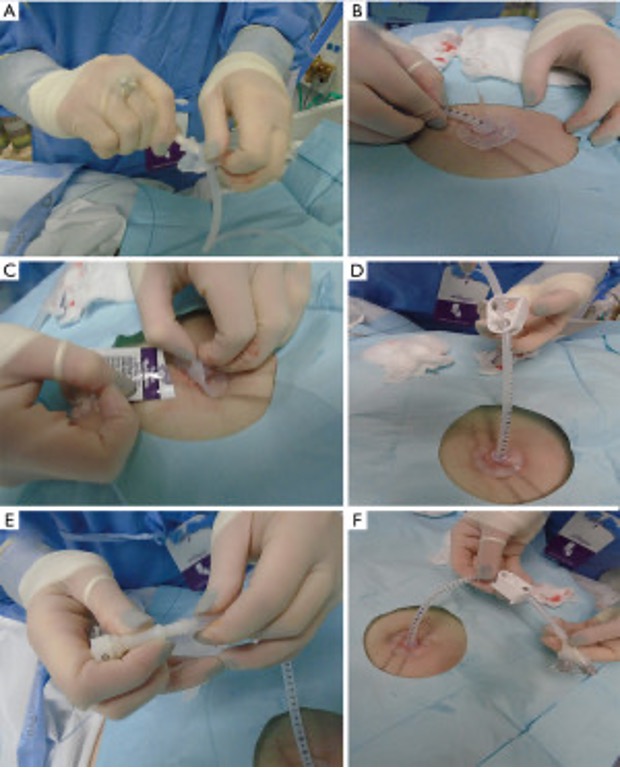
The more durable gastrostomy tube options are those that go directly into the stomach via the abdominal wall. These tubes can be placed in a variety of ways. Interventional radiologists use a “push technique” to position tubes percutaneously, using only fluoroscopic guidance, in a radiology suite or at bedside under conscious sedation,6,8 although some practitioners offer CT-guided8,9 or ultrasound-based5,8,9 placement. PEG tubes are placed via esophagogastroduodenoscopy (EGD), usually at the bedside under conscious sedation, by gastroenterologists, surgeons, or intensivists using either a “pull technique” (Figures 1 and 2) or a “push technique,” depending on available supplies and proceduralist preference.5,8,9 Under general anesthesia, surgeons can place PEG tubes laparoscopically with or without EGD assistance (using either the pull or push technique), or by an open approach (i.e., in conjunction with another operation).8
Figure 1. Placement of the PEG tube via the pull method
Figure 2
Image Source: Wei M, Ho E, Hegde P. An overview of percutaneous endoscopic gastrostomy tube placement in the intensive care unit. J Thorac Dis. 2021;13(8):5277-5296.
Risk Factors and Alternative Approaches
In many locations, operating room access is limited, so bedside procedure options present a timely, cost-efficient alternative, avoiding the risk of general anesthesia. PEG tube candidates are typically screened for ability to tolerate an EGD (i.e., many patients with proximal aerodigestive tract malignancies are not candidates because of the presence of obstructing tumor and/or esophageal stricture) as well as safety for the percutaneous transabdominal approach. Obesity, adhesive disease from prior surgeries (especially of the foregut), and atypical location of the stomach relative to the small bowel or colon can increase the risk of complications associated with bedside PEG placement; these patients may instead be offered laparoscopic or open gastrostomy tube placement.7,8,10,11 In this case, the patient had none of these risk factors, so there was no missed opportunity for prevention before placement.
For any patient undergoing PEG placement, the gastrostomy site is determined using indirect confirmatory adjuncts such as:
- External landmarks to the left of the patient’s midline confirmed by:
- transillumination of the abdominal wall via the endoscope at the planned insertion site, and
- 1:1 indentation of the stomach seen with the endoscope at the externally palpated site of planned insertion7,8
- A “safe track” technique with endoscopic visualization of a fine 25-gauge “finder” needle puncturing the stomach at the expected location with bubbles noted in the syringe immediately as the needle enters the stomach. If bubbles are detected before the needle is visualized in the stomach, it may have traversed overlapping bowel.12,13,14
If transillumination fails and a safe puncture site cannot be located using the above techniques, then a PEG tube can be inserted via surface ultrasound-guided landmarks,15 percussion of the liver edge,14 or using preoperative air insufflation to guide abdominal plain film marking of the puncture site.16
In this patient’s case, abdominal wall transillumination was documented, and one-to-one motion of the stomach occurred with external palpation.
PEG placement has an overall complication rate from 9% to 36%, depending on the classification of complications and the time period for ascertainment.11,14,17,18 The most common complications are wound problems such as infection (5-65%)6 and leakage from the stoma (1-2%),6 tube malfunction,19 aspiration of tube feedings and/or pneumonia,6,11 bleeding from the abdominal wall or gastric wall ulceration,6,11,14 tube dislodgement with peritonitis or necrotizing soft tissue infection,6,11,14 injury to adjacent organs such as the colon,17 small bowel, liver, spleen,20 or esophagus,14 tumoral seeding of the PEG tract (<1%),6 and gastrostomy site herniation.6,9 “Buried bumper” syndrome occurs in about 1-4% of cases6,9,14 when the PEG tube’s internal bumper causes focal pressure necrosis on the gastric and/or abdominal wall, leading to erosion and “burial” under the skin, potentially outside the gastric lumen. Liver4,10,13-15,21-25 or spleen injury20 are exceedingly rare complications of PEG placement, with one report citing only 16 cases of transhepatic placement reported in the literature, although these complications may be underreported due to the lack of specific symptoms and the need for imaging for diagnosis.10
In this case, failure to recognize the transhepatic PEG tube location on the initial postoperative CT scan contributed to the unintentional blind removal of the malpositioned tube. Although not the focus of this commentary,27 diagnostic errors have received growing attention over the past 15 years as providers work to improve patient safety. Delayed or missed diagnoses comprise the most common and costly reasons for malpractice claims,28 with radiology being one of the specialties most susceptible to claims of medical negligence due to “failure to diagnose.”29 In the radiology literature, these diagnostic errors have been categorized as pre-reporting, reporting, or post-reporting errors.30 In this case, the reporting error can be further classified as a “perceptual error,” whereby the relevant finding was not noticed but is visible in retrospect.30 A widely accepted classification scheme developed by Kim and Mansfield assigns diagnostic errors into 12 groups based on the cause: 1) false-positive or over-reading, 2) faulty reasoning, 3) lack of knowledge, 4) under-reading, 5) poor communication, 6) technique-related, 7) prior examination-related, 8) history-related, 9) location-related, 10) satisfaction of search, 11) complication, and 12) satisfaction of report.29 The missed finding of the transhepatic PEG tube in this case can be linked to several potential categories:
- under-reading error, in which the abnormal finding was “undeniable and detectable”29 yet completely missed;
- history-related error, where the indications for the study only included the phrase “intractable emesis... evaluate for small bowel obstruction,” without mention of recent PEG placement;
- location-related error, where the abnormality was outside the anatomic area of focus (i.e., runoff of oral contrast through small bowel);
- satisfaction of search, in which accompanying findings not specific to the primary question are under-read because the primary inquiry was satisfied.
Most hospitals utilize some form of peer review to identify care process and system solutions that can be implemented to enhance radiologists’ performance. An online survey of 339 institutions including 61 teaching hospitals confirmed that most facilities have a least one method of peer review; many sites use proactive review (double interpretation by separate radiologists) in addition to reactive methods (case review triggered when a discrepancy has been noted).26 Some authors have reported on the challenges in developing software and technology to make peer review easier to accomplish and to reduce reviewer variability.31
Although it is impossible to prevent all trans-hepatic PEG tube placements, one article suggests real-time ultrasound visualization of the intended PEG tract4 and another cautions against elevating the head of the patient’s bed too steeply (more than 80 degrees) to avoid excessive caudal displacement of the liver edge below the costal margin.25
When a PEG tube complication has been identified, options for management include treating the complication while leaving the tube in place, removing the PEG tube without replacing it, or exchanging it or replacing it via the same or a different approach.4,10,11,14 The management and timing of the intervention is dictated by the patient’s clinical status and the specific complication. If the patient no longer requires a PEG tube for nutritional support, it can be removed. This is usually done in a clinic setting without sedation or specialized equipment. When the PEG tube has complications, such as a skin or soft tissue infection, but feeding access is still required, the tube can sometimes be used while managing the complication. When the patient is not tolerating gastric feeding or is having aspiration events, the PEG tube can be exchanged or replaced or converted to a gastrojejunostomy tube under fluoroscopic or surgical guidance. Generally, malpositioned tubes (which most commonly pass through a hollow viscus such as small bowel or colon) are managed by either laparoscopic or open revision. With transhepatic positioning, removal or revision of the PEG tube may be deferred if the tube is functioning and the potential risk of operative intervention exceeds the risk of utilizing the malpositioned tube.4,10,11,14,25
There are several methods for removing PEG tubes. These include:
- the somewhat controversial “cut & push” method,32 whereby a PEG tube <18 French (Fr) in well-selected patients,33,34 or 20-24 Fr35 in other settings, is cut at the level of the skin and the internal gastric bumper is allowed to migrate downstream and pass through the colon, and passage of the bumper is confirmed either clinically34,35 or with abdominal radiographs33;
- the traction technique where the PEG tube is pulled out of the stomach through the abdominal wall36;
- the endoscopic removal technique where the PEG tube is cut at the level of the skin but the internal gastric bumper is extracted endoscopically via EGD from the stomach through the mouth;19 and
- laparoscopic or open surgical approaches if the PEG tube needs to be removed or revised under direct visualization, allowing inspection of the involved and/or adjacent organs, potentially including infected tissue or other complications.3,4,33
It is widely accepted that removing a PEG tube less than one month after placement, regardless of technique, subjects the patient to an unacceptable risk of peritonitis and need for surgery.21 In this patient’s case, the surgical team was not made aware of the patient’s inability to tolerate tube feedings less than a week after the PEG was placed, which might have prompted an early postoperative CT scan to investigate the PEG site and pathway. A CT scan was ordered by the primary service about 2 weeks postoperatively without surgeon input, but the malpositioned tube was not identified and the surgical team was not alerted. Since the patient was not tolerating gastric feeding, but still required access, the typical surgical approach would be an operative revision, especially in this case where the PEG was less than a month old. Even without dual antiplatelet therapy, the risk of causing the liver to bleed with removal of the malpositioned PEG tube via the traction technique would be considered excessive. In this case, the PEG was removed via traction technique about 4 months after its placement. The risk of bleeding with transhepatic tubes varies according to the position of the PEG tube. A PEG tube traversing the periphery of the liver where there are smaller blood vessels and less parenchyma involved is associated with lower risk of significant bleeding than a centrally malpositioned PEG tube, where there are larger blood vessels and more parenchyma involved.3,4,21,25
Approach to Improving Patient Safety
Patients who require anticoagulant and/or antiplatelet therapy to minimize thrombotic risk are at increased risk for postoperative bleeding complications. Timing of discontinuation of anti-platelet therapy after invasive procedures is based on an individualized risk-benefit analysis, weighing the risk of thrombotic events against the risk of bleeding for the anticipated procedure.37 In the case reviewed here, the neurosurgeon deemed the patient to be at prohibitively high risk of stroke if her anti-platelet therapy was interrupted before two months, which was later extended to three months. The extension of anti-platelet therapy delayed the anticipated surgical intervention for the malpositioned transhepatic PEG tube, which contributed to the change in the original management plan.
It has been well-established that care transitions across multiple providers and locations of care are associated with increased risk of patient harm, usually due to loss of information or other problems in communication.38-42 Contributing factors include challenges in transmitting consistent messages about patient status and care plans via verbal, written, and/or electronic health record (EHR) systems. Additional complicating factors include various care locations, especially when multiple care teams are involved. Another recognized vulnerability is through transitions in environments of care, such as from inpatient to subacute rehabilitation care, where interfacility data transmission remains highly susceptible to loss or degradation.40-44
With the widespread recognition that care transitions are an extremely vulnerable time for patients, many clinicians have worked to develop mechanisms to mitigate this risk.38,42 Current best practices include using checklists at hand-offs, implementing standardized hand-off tools, and standardizing the content of discharge summaries regarding follow-up appointments and procedures.40-45 More than ever, clinical teams are working together to determine how to communicate clearly and directly with patients and their families and support networks.38,40,42,46-48
A Commonwealth Fund report noted, “The need for coordination is in proportion to the degree of care fragmentation and the complexity of a patient's situation. Coordination of patient care is so important, and often must be achieved at so many points of potential breakdown, that multiple modes are needed. These might include the processes and outcomes of building pathways and protocols, oversight committees to achieve consistency and coordination of care, and clinical nurse specialists and other specialized integrative roles.”49
In this case, the surgical division could consider both individual and system process improvement options, including:
- At the individual level, surgical team members can develop a process to regularly enter information into the EHR (or a team list) about patients with long inpatient courses and complex post-discharge planning needs. This information can be propagated through progress notes and included on discharge summaries, discharge orders, and discharge instructions that are provided to patients.
- At a systems level, the creation of a shared patient list in the EHR may bolster inter-service communication. This list should be accessible to faculty, residents, and advanced practice practitioners (APPs), in all locations where they provide care. With shared lists, it may be possible to auto-populate surgery-specific details into non-surgical team notes and documentation, such as discharge summaries, orders, and instructions.
- A third systems solution might be to minimize the number of teams that offer percutaneous enteral tube placement in a facility, possibly through the creation of a single multidisciplinary “nutrition support team” which assesses patients for endoscopically placed feeding tubes and can standardize perioperative care delivery so that admitting/attending teams have a single entity to contact for any feeding access consults and/or complications and follow-up.50 Such a multidisciplinary team concept could include interventional radiologists in some facilities.
- A final comprehensive system option is the development of team-based patient navigation services, as previous authors have described for polytrauma47 or traumatic brain injured patients. In this system, a navigator and the multidisciplinary team coordinate care between patient and family, care providers, and ancillary support agencies and resources.48
Take-Home Points
- PEG tubes are frequently placed and generally safely inserted in well-selected candidates. Health care providers and care teams should be aware of basic strategies to recognize and handle PEG complications.
- Any identified complications should be documented clearly for subsequent provider awareness. Because patient care handovers involving multiple specialists in multiple care environments have been shown to increase the risk of adverse events, care teams should implement tactics to minimize the risk of adverse events.
- Best practices to mitigate patient care transition risks are to minimize the number of provider transitions and the number of care teams performing gastrostomy procedures where possible, to standardize communication tools and strategies, and to optimize individualized post-discharge care instructions.
- Continuity of care guidance should be communicated clearly in face-to-face interactions supported by the EHR. Specific follow-up plan details should be included in the consultant sign-off note and also in the primary team’s discharge summary.
- Teams practicing in a service model should consider creating and utilizing a clinic “follow-up” EHR list with the responsible provider and follow-up recommendations. This patient list should be readily available to faculty, resident, and APP providers in clinic as well as in the inpatient environment.
Rachel A. Hight, MD, FACS
Associate Professor
Associate Program Director, General Surgery Residency Program
Department of Surgery, Division of Trauma, Acute Care Surgery and Surgical Critical Care
UC Davis Health
rhight@ucdavis.edu
References
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872-875. [Available at]
- Gauderer MW. Percutaneous endoscopic gastrostomy-20 years later: a historical perspective. J Pediatr Surg. 2001;36(1):217-219. [Available at]
- Hucl T, Spicak J. Complications of percutaneous endoscopic gastrostomy. Best Pract Res Clin Gastroenterol. 2016;30(5):769-781. [Available at]
- Chhaparia A, Hammami MB, Bassuner J, et al. Trans-hepatic percutaneous endoscopic gastrostomy tube placement: a case report of a rare complication and literature review. Gastroenterology Res. 2018;11(2):145-149. [Free full text]
- Wei M, Ho E, Hegde P. An overview of percutaneous endoscopic gastrostomy tube placement in the intensive care unit. J Thorac Dis. 2021;13(8):5277-5296. [Free full text]
- Liu X, Yang Z, He S, Wang G. Percutaneous endoscopic gastrostomy. Int J Gastrointest Interv. 2021;10:42-48. [Free full text]
- Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R, et al. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol. 2014;20(24):7739-7751. [Free full text]
- Rajan A, Wangrattanapranee P, Kessler J, et al. Gastrostomy tubes: fundamentals, periprocedural considerations, and best practices. World J Gastrointest Surg. 2022;14(4):286-303. [Free full text]
- Gawande RS, Bailey CR, Jones C, et al. MDCT evaluation of complications of percutaneous gastrostomy tube placement. Emerg Radiol. 2019;26(6):663-674. [Available at]
- Imam Z, Simons-Linares CR. Transhepatic Insertion of Percutaneous Endoscopic Gastrostomy Tube. Case Rep Gastrointest Med. 2020;2020:4516032. [Free full text
- Pih G, Na H, Ahn J, et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy insertion. BMC Gastroenterol. 2018;18(1):101. [Free full text]
- Foutch PG, Talbert GA, Waring JP, et al. Percutaneous endoscopic gastrostomy in patients with prior abdominal surgery: virtues of the safe tract. Am J Gastroenterol. 1988;83(2):147-150. [PubMed citation]
- Bichile T, Aung T, Vainder J. A rare complication of PEG tube placement. Practical Gastroenterology. 2014; 80-83. [Free full text]
- Boeykens K, Duysburgh I. Prevention and management of major complications in percutaneous endoscopic gastrostomy. BMJ Open Gastro 2021;8:e000628. [Free full text]
- Tomaszewski M, Strohl M, Wong P. Transhepatic PEG tube placement. Clin Gastroenterol Hepatol. 2020;18(9):e101. [ Available at]
- Chang WK, Hsieh TY. Safety of percutaneous endoscopic gastrostomy in high-risk patients. J Gastroenterol Hepatol. 2013;28 Suppl 4:118-122. [Free full access]
- Trenschel R, Geraghty F, Mirza J, et al. Percutaneous endoscopic gastrostomy misplacement in the transverse colon of a neurocognitively compromised patient. Cureus. 2022;14(2):e22063. [Free full text]
- Turan UF, Katar MK. Evaluation of 644 percutaneous endoscopic gastrostomy patients in a single center. Cureus. 2023;15(4):e38324. [Free full text]
- Shah R, Shah M, Aleem A. Gastrostomy Tube Replacement. In:StatPearls. Treasure Island (FL): StatPearls Publishing; April 17, 2023. [Free full text]
- Patel BB, Andrade C, Doraiswamy V, et al. Splenic avulsion following PEG tube placement: a rare but serious complication. ACG Case Rep J. 2014;2(1):21-23. [Free full text]
- Gubler C, Wildi SM, Bauerfeind P. Liver injury during PEG tube placement: report of two cases. Gastrointestinal Endoscopy. 2005; 61(2):346-348. [Available at]
- Fyock CJ, Kethu SR. PEG placement causing liver perforation. J Clin Gastroenterol. 2009;43(4):385. [Available at]
- Shaw J, Casey K. A PEG tube through the liver. Am J Gastroenterol. 2009;104(5):1323-1324. [Available at]
- Poggi G, Montagna B, Cesare P. Transhepatic percutaneous endoscopic gastrostomy. Open Journal of Gastroenterology. 2013;3(2):87-88. [Free full text
- Picazo-Ferrera K, Escobedo-Paredes DM, Herrera-Servín MA, et al. Incidental transhepatic placement of a percutaneous endoscopic gastrostomy tube. Presentation of a rare complication and a literature review. Rev Gastroenterol Mex (Engl Ed). 2020;85(4):479-481. [Free full text]
- Kaewlai R, Abujudeh H. Peer review in clinical radiology practice. AJR Am J Roentgenol. 2012;199(2):W158-W162. [Free full text]
- Sarkar U, Shojania K. Annual Perspective 2014: Diagnostic Errors. March 2015. AHRQ Patient Safety Network. Accessed November 6, 2023. [Free full text]
- Pinto A, Brunese L. Spectrum of diagnostic errors in radiology. World J Radiol. 2010;2(10):377-383. [Free full text]
- Onder O, Yarasir Y, Azizova A, et al. Errors, discrepancies and underlying bias in radiology with case examples: a pictorial review. Insights Imaging. 2021;12(1):51. [Free full text]
- Brady AP. Error and discrepancy in radiology: inevitable or avoidable? Insights Imaging. 2017 Feb;8(1):171-182. [Free full text]
- Strickland NH. Quality assurance in radiology: peer review and peer feedback. Clin Radiol. 2015;70(11):1158-1164. [Free full text]
- Pratt J, Green S. Removal of percutaneous endoscopic gastrostomy tubes in adults using the "cut and push" method: a systematic review. Clin Nutr ESPEN. 2017;21:59-65. [Available at]
- Korula J, Harma C. A simple and inexpensive method of removal or replacement of gastrostomy tubes. JAMA. 1991;265(11):1426-1428. [Available at]
- Kejariwal D, Bromley D, Miao Y. The "cut and push" method of percutaneous endoscopic gastrostomy tube removal in adult patients: the Ipswich experience. Nutr Clin Pract. 2009;24(2):281-283. [Available at]
- Agha A, AlSaudi D, Furnari M, et al. Feasibility of the cut-and-push method for removing large-caliber soft percutaneous endoscopic gastrostomy devices. Nutr Clin Pract. 2013;28(4):490-492. [Available at
- Melling G, Farley J. Complication rates associated with traction removal of percutaneous endoscopic gastrostomy tubes. British J of Nursing. 2022; 31(7): S22-S26 [Available at]
- Moster, M., Bolliger, D. Perioperative guidelines on antiplatelet and anticoagulant agents: 2022 update. Curr Anesthesiol Rep. 2022;12:286–296. [Free full text]
- McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville (MD): Agency for Healthcare Research and Quality (US); 2007 Jun. Report No.: 04(07)-0051-7. [Free full text]
- Ong MS, Coiera E. A systematic review of failures in handoff communication during intrahospital transfers. Jt Comm J Qual Patient Saf. 2011;37(6):274-284. [Available at]
- Patel SJ, Landrigan CP. Communication at transitions of care. Pediatr Clin North Am. 2019 Aug;66(4):751-773. [Available at]
- Abraham J, Nguyen V, Almoosa KF, et al. Falling through the cracks: information breakdowns in critical care handoff communication. AMIA Annu Symp Proc. 2011;2011:28-37. [Free full text]
- Siefferman JW, Lin E, Fine JS. Patient safety at handoff in rehabilitation medicine. Phys Med Rehabil Clin N Am. 2012;23(2):241-257. [Available at]
- Sarzynski E, Hashmi H, Subramanian J, et al. Opportunities to improve clinical summaries for patients at hospital discharge. BMJ Qual Saf. 2017;26(5):372-380. [Available at]
- McFadden NR, Gosdin MM, Jurkovich GJ, et al. Patient and clinician perceptions of the trauma and acute care surgery hospitalization discharge transition of care: a qualitative study. Trauma Surg Acute Care Open. 2022;7(1):e000800. [Free full text]
- O'Leary KJ, Liebovitz DM, Feinglass J, et al. Creating a better discharge summary: improvement in quality and timeliness using an electronic discharge summary. J Hosp Med. 2009;4(4):219-225. [Available at]
- Koenig CJ, Maguen S, Daley A, et al. Passing the baton: a grounded practical theory of handoff communication between multidisciplinary providers in two Department of Veterans Affairs outpatient settings. J Gen Intern Med. 2013;28(1):41-50. [Free full text]
- Hall EC, Tyrrell RL, Doyle KE, et al. Trauma transitional care coordination: a mature system at work. J Trauma Acute Care Surg. 2018;84(5):711-717. [Available at
- Rosario ER, Espinoza L, Kaplan S, et al. Patient navigation for traumatic brain injury promotes community re-integration and reduces re-hospitalizations. Brain Inj. 2017;31(10):1340-1347. [Available at]
- March A. Perspective: Consistency, Continuity, and Coordination – the 3C’s of Seamless Patient Care. The Commonwealth Fund. Area of Focus: Improving Health Care Quality. 2006. Accessed November 6, 2023. [Free full text]
- Westaby D, Young A, O'Toole P, et al. The provision of a percutaneously placed enteral tube feeding service. Gut. 2010;59(12):1592-1605. [Available at]