Management of CSF Leaks After Elective Spine Surgery: Routine Laminectomy Leads to Fatal Discitis and Sepsis
Castillo JA, Price R, Kim KD. Management of CSF Leaks After Elective Spine Surgery: Routine Laminectomy Leads to Fatal Discitis and Sepsis. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Castillo JA, Price R, Kim KD. Management of CSF Leaks After Elective Spine Surgery: Routine Laminectomy Leads to Fatal Discitis and Sepsis. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2024.
Disclosure of Relevant Financial Relationships: As a provider accredited by the Accreditation Council for Continuing Medical Education (ACCME), the University of California, Davis, Health must ensure balance, independence and objectivity in all its CME activities to promote improvements in health care and not proprietary interests of a commercial interest. Authors, reviewers and others in a position to control the content of this activity are required to disclose relevant financial relationships with ineligible companies related to the subject matter of this educational activity. The Accreditation Council for Continuing Medical Education (ACCME) defines an ineligible company as “as any entity whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients” and relevant financial relationships as “financial relationships in any amount occurring within the past 24 months that create a conflict of interest.
Kee D. Kim, MD and Richard Price, MD disclosed a relevant financial disclosure with ineligible companies related to this CME activity which have been mitigated through UC Davis Health, Office of Continuing Medical Education procedures to meet ACCME standards.
| NAME | COMPANY | RELATIONSHIP |
|---|---|---|
| Kee D. Kim, MD | AbbVie | Research Funding |
| Empirical Spine | Research Funding | |
| Ferring | Consultant | |
| Globus | Consultant | |
| GS Medical | Consultant | |
| Highridge | Consultant, Royalties / Patent Beneficiary | |
| Medtronic | Research Funding | |
| Molecular Matrix | Ownership Interest (stock/stock options) | |
| Precision Spine | Consultant, Royalties / Patent Beneficiary | |
| Seikagaku | Consultant | |
| Stryker | Research Funding | |
| Richard Price, MD | Augmedics | Consultant |
| FYR Medical | Ownership Interest (stock/stock options) | |
| Stryker | Consultant |
Debra Bakerjian, PhD, APRN, RN; Jose A Castillo Jr, MD; Shelley Palumbo, SLP; Patrick Romano, MD, MPH; Garth Utter, MD, MSc, FACS for this Spotlight Case and Commentary have disclosed no relevant financial relationships with ineligible companies related to this CME activity.
Learning Objectives
At the conclusion of this case, participants should be able to:
- Identify the clinical manifestations of a cerebrospinal fluid (CSF) leak.
- Describe the workup to diagnose a CSF leak.
- List conservative and non-conservative measures to manage a CSF leak to prevent serious complications.
The Case
A 70-year-old man underwent a L4-5 decompressive lumbar laminectomy and discectomy. The surgery was complicated by an intraoperative durotomy. Immediately after the operation, the surgeon informed the patient and instructed him to lie flat on his back for several days. The patient was subsequently discharged home. On postoperative day (POD) 6, the patient reported clear drainage from the surgical site. He was advised to continue to lie flat on his back until the drainage stopped. By POD 12, the patient noted ongoing clear fluid drainage, now accompanied by positional headaches. After re-evaluation by the spine surgical team, he was instructed to continue lying flat and to return the following day for further assessment. At the follow-up visit, the surgeon suspected a cerebrospinal fluid (CSF) leak based on examination. Bed rest was extended for another 5 days. However, drainage persisted, prompting further observation and a course of oral antibiotics.
On POD 22, at a subsequent clinic follow-up, the patient reported intermittent chills, continued drainage, and pain at the surgical site. Examination by the surgical team revealed a wound defect with "scant white/yellow liquid." Wound care was initiated with home health nurses performing wet-to-dry dressing changes. Peroxide-soaked sterile cotton was applied to the drainage site after manual expression of discharge. On POD 25, the wound appeared to have dehisced, and the patient was readmitted to the hospital for advanced wound care and intravenous antibiotics. On POD 29, the wound was explored but no dural defect was found. Intraoperative cultures were negative for bacteria. The patient was discharged, but his condition worsened over the following week, with increasing back pain. On POD 42, the patient was readmitted and diagnosed after magnetic resonance imaging (MRI) with discitis and osteomyelitis. He was diagnosed with sepsis, which did not respond to escalating antibiotic treatment. On POD 50, he died after an episode of aspiration and cardiopulmonary arrest.
The Commentary
By Jose A Castillo Jr, MD, Richard Price, MD, PhD, Kee D Kim, MD
Introduction
Cerebrospinal fluid (CSF) leaks are a serious complication that can arise from injury to the dura, which is a thick fibrous membrane surrounding the brain and spinal cord. Most CSF leaks are iatrogenic, due to a dural tear during spinal surgery or anesthesia. With advances in surgical technology, spine surgeons have several options to manage intraoperative durotomies. CSF leaks also have varying degrees of severity. When the extravasated CSF forms a fibrous capsule beneath the skin, it is classified as a pseudomeningocele.1,2 When the CSF develops a direct connection with the outside environment, as in this case, it is referred to as a CSF fistula with CSF leak.3 Persistence of such a fistula may provide a direct conduit for bacteria to enter the meninges, causing meningitis, and the deep surgical wound, causing discitis or vertebral osteomyelitis. Intraoperative treatment of durotomies using various materials as well as postoperative CSF leak management strategies will be discussed below. In the patient described in this case, more timely and effective management of the CSF leak might have prevented catastrophic infectious complications.
Background
Epidemiology
Intraoperative durotomy can occur incidentally or intentionally. When accessing intradural tumors, the dura is opened intentionally, and reapproximating via suturing is the intended plan for dural closure. If water-tight dural closure is not achieved, the risk of postoperative CSF leak is increased. Incidental durotomies often result in complex tears that may go unnoticed. Intraoperative, incidental durotomies are a common occurrence during lumbar laminectomy.4,5,6,7 Rates of incidental durotomy have been reported in the literature to occur from 0.5% in lumbar discectomy to 35% in a single retrospective study.8,9
Pathophysiology
Intraoperative durotomy, by definition, requires a dural tear. If cerebrospinal fluid (CSF) leaks through the dural tear into the surrounding tissue, forming a fibrous capsule, the condition is termed a pseudomeningocele (Figure 1).1,2,10
Figure 1. Layers involved in the formation of a pseudomeningocele, with schematic representation of nerve root entrapment at the site of dural defect
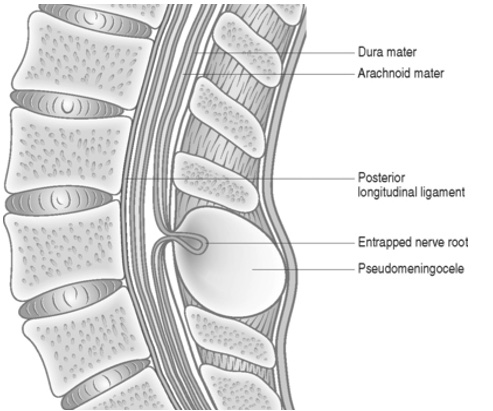
Pseudomeningoceles do not communicate with another cavity or the outside world. If the extradural fluid communicates with another cavity, such as the pleura, then it is classified as a CSF fistula. If a direct communication to the outside of the body exists, then it is considered a CSF fistula and CSF leakage can be appreciated from a wound.3
There is ongoing debate regarding the factors that contribute to persistent CSF fistula formation and leakage. CSF extravasation into a pseudomeningocele, if small, can be self-limited.11 However, if enough CSF collects in the traumatized paraspinal musculature, an abnormal connective tissue reaction can occur, hindering CSF reabsorption and leading to wound breakdown.12,13 If watertight closure of dura is not achieved, then firm fascial closure may reduce the risk of CSF leak. In this setting, increased epidural CSF pressure prevents CSF extravasation through the dural defect by creating an unfavorable pressure gradient. If not tightly reapproximated, dural flaps cannot remain adhered at the durotomy site and CSF leak is more likely.14 Both mechanisms—CSF extravasation into a pseudomeningocele and inadequate fascial closure— may contribute to the formation of pseudomeningoceles and CSF fistulas.
Table 1. Signs and Symptoms Associated with Pseudomeningoceles and CSF Fistulas
|
|
| Source: Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2000;9(1):e5. [Free full text] | |
Clinical Manifestations
Intraoperative durotomy can manifest in various ways.3 A common sign during surgery is the pooling of clear CSF in the surgical field. Postoperatively, more severe complications may arise, such as wound dehiscence, persistent pseudomeningocele formation, meningitis, hydrocephalus, arachnoiditis, and, in rare cases, death.15 These complications may emerge immediately after surgery or even years later.1
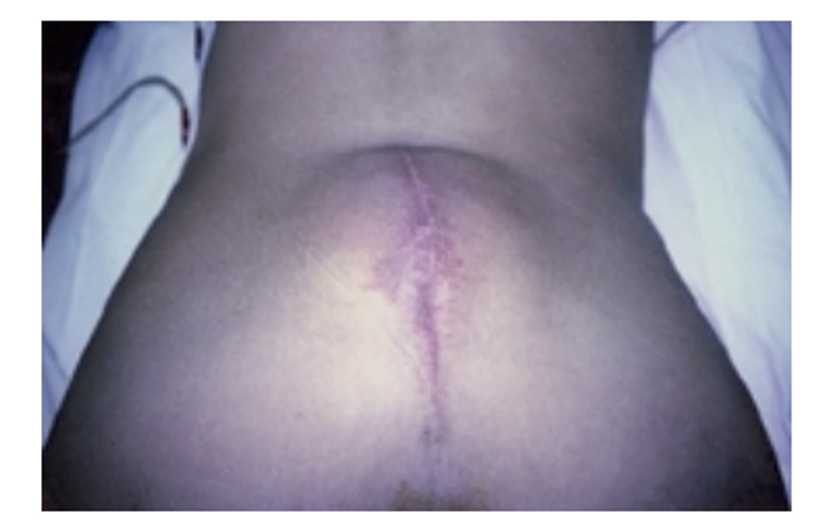
Pseudomeningoceles typically appear as fluctuant masses that may become more pronounced with Valsalva maneuvers (Figure 2).3,16 Pain can sometimes be elicited by palpation of the pseudomeningocele.17 CSF leaks can be appreciated on inspection, as wound openings with clear and colorless fluid drainage.18 Some patients report recurrence of their preoperative symptoms, ranging from radiculopathy to myelopathy (i.e., spinal cord injury), presumably due to herniation of nerve tissue.6,13,17,19–27 Patients may also present with intracranial hypotension symptoms that include photophobia, cranial nerve palsies, and tinnitus.28,29
Figure 2. Photograph of a patient in the prone position, revealing a large fluctuant mass consistent with pseudomeningocele in the posterior lumbar location.
Diagnosis
MRI
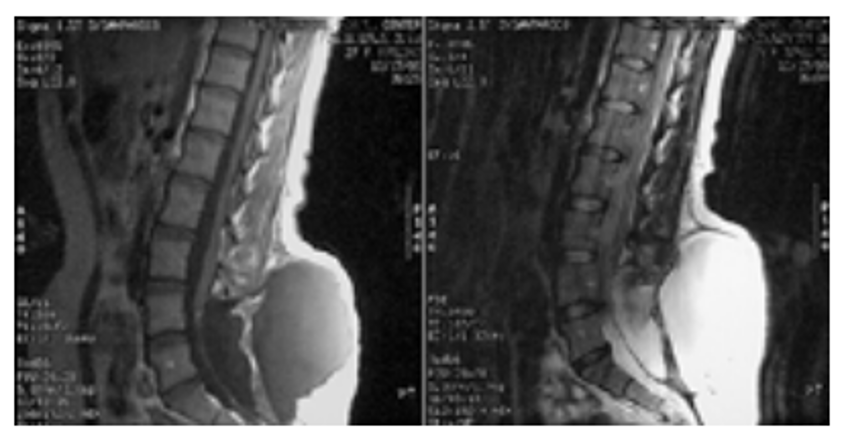
Diagnosis is confirmed via spinal MRI with history and physical examination to narrow the differential. A region of hypointensity on T1 weighted images and hyperintensity on T2 weighted images, consistent with a fluid collection, is seen (Figure 3).25,30 MRI can also help delineate the location of communication with the dura.
Figure 3. Sagittal T1 (left) and T2 (right) weighted MRI showing a large dorsal fluid collection contiguous with the subarachnoid space.
Beta-2 Transferrin
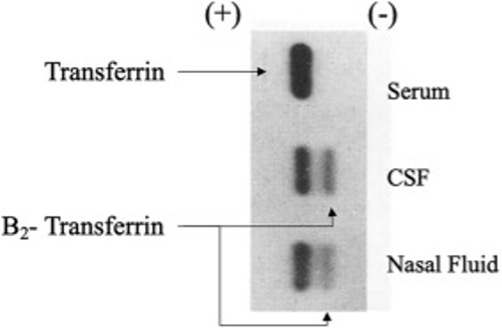
If a CSF leak is present and clear fluid is visible from a wound defect, the fluid can be analyzed for beta-2 transferrin, which is a protein found only in CSF.31 Less than 1 mL is needed to test for its presence. By comparison, serum and other body fluids have only a beta-1 transferrin band (Figure 4).32
Figure 4. Nasal Fluid Positive for Beta-2 Transferrin Suggesting CSF Source
Intraoperative Management
When a dural violation is recognized, primary closure should be attempted to prevent future complications. The goal is “watertight” closure of the dura,33 but this goal can be difficult to achieve, especially for incidental intraoperative durotomies, as the tear can occur at an inaccessible location. For this reason, most argue that it is sufficient to contain neural elements and prevent pseudomeningoceles or CSF fistulas/leaks. If a tear is not accessible, then observation, sealants, or CSF diversion maneuvers can be used.34,35 It is helpful to organize primary repair strategies into 3 categories: sutures, sealants, and patches/grafts.8,36–40 Currently, no official consensus exists on the ideal approach.
Sutures
Four commonly used suture materials include nylon, polypropylene, polytetrafluoroethylene, and silk. All these materials are non-absorbable and elicit minimal to no acute inflammatory reaction, which is the goal in this setting.22,41 One study found that in a small linear incision, simple interrupted silk sutures were superior to running, running locked, or interrupted vertical mattress techniques.42 However, another study found no difference in the risk of CSF leak between simple running and interrupted running sutures with polypropylene.43
A biomechanical study found that a mean CSF peak pressure of 34 cm H2O can be observed before CSF leakage with polytetrafluoroethylene, compared with only 21 cm H2O for nylon. This superiority was consistent across suturing techniques.44 Another study showed that polypropylene had a higher mean pressure threshold than nylon in cadaveric specimens.45 No studies currently exist that directly compare polytetrafluoroethylene and polypropylene. Ultimately, deciding on the best suture material or technique remains challenging. We advocate for using the material with which one is most comfortable and has had the most success, as surgeon experience has been shown to favorably impact outcomes.46
After completing a primary closure, the surgeon should test their suturing for persistent CSF leak using a Valsalva maneuver (VM), where the anesthesiologist increases intrathoracic pressure. The VM can be performed by switching the ventilator to manual ventilation, and with the adjustable pressure-limiting valve fully closed, the anesthesiologist increases fresh gas flow and squeezes the breathing circuit bag for 15-20 seconds to achieve an intrathoracic pressure around 40 mmHg.47 One should be mindful that rare complications of this maneuver have been reported, ranging from stroke and retinopathy to pneumothorax.47 Clinical judgment should prevail when deciding whether to perform a VM.
Patches/Grafts
If primary closure of an intraoperative durotomy is not feasible, because the location is too lateral or neural elements would become compressed, then dural patches/grafts can be employed. Ideally, the patch/graft material should restore the continuity of the dura mater, minimize CSF leak, mimic the compliance of dura, and be biologically inert.48 Currently, the options fall into four categories: autograft, allograft (i.e., cadaveric dura), xenograft (animal-derived), and synthetic materials. In the autologous category, the options typically used in spinal surgery include fascia lata and muscle.
One of the most frequently used products is a porous type 1 collagen matrix manufactured from bovine deep flexor tendon and engineered to remove antigenic components.49 An interesting property of this material is that it has some secondary hemostatic capabilities as its porosity allows platelets and fibroblasts to infiltrate it and create fibrin clots and lay natural collagen fibers, which are believed to aid in preventing CSF leakage and aiding dural repair. This product also resorbs completely within 1 year,50 with no inflammatory response, a 1.9% infection rate, and a 2.1% leakage rate.51
Sealants
If a CSF leak persists after attempted primary closure with a patch or graft, a sealant can be placed over the area of the leak. Despite animal studies suggesting sealant alone can withstand high hydrostatic pressures,52–54 some remain in situ for only 10-14 days;55 thus, sealants alone may be inadequate but can be used to supplement a patch, graft, or suture repair.
Commercially available sealants fall into two categories as either synthetic, consisting of polyethylene glycol (PEG)-based polymers, or fibrin-based from allogenic or autogenic fibrinogen combined with thrombin and other hemostatic factors. Synthetic, PEG-based sealants work as hydrogels, absorbing fluid and swelling to minimize mass on the spine.56,57 One prospective, multicenter, randomized controlled trial comparing a synthetic sealant to standard-of-care closure during spinal surgery showed that the sealant improved the percentage of patients with intraoperative watertight closure (99% versus 79%).58 In a larger, nonrandomized, two-armed prospective study, the same product was confirmed as safe, although its 90-day outcomes did not significantly differ from those of other products.59 Only this PEG-based sealant has been approved by the Food and Drug Administration (FDA) as a secondary agent for dural closure in the spine.60
Postoperative Pseudomeningocele/CSF Fistula/Leak Management
Conservative Approach
If an intraoperative spinal durotomy cannot be closed “watertight” or the patient exhibits symptoms, preventing pseudomeningocele formation or controlling the CSF leak depends on manipulating differences in subarachnoid and epidural pressure.61 To this end, Trendelenburg positioning is traditionally prescribed after lumbar dural tears62 because the upright position increases the hydrostatic pressure of the CSF and may worsen leakage.63 Interestingly, the data supporting this approach are limited to series describing patients successfully treated with prolonged bed rest without a control group of alternative options.64 A recent meta-analysis reported that early mobilization reduced pulmonary complications, when compared with prolonged bed rest, among patients who underwent primarily closed durotomies. No significant difference in the incidence of CSF leak or the need for additional surgical durotomy repair was appreciated. Surgeons should use bed rest and Trendelenburg positioning with caution, given the risk of postoperative complications.65 Nonetheless, several surveys have shown that up to 89% of spine surgeons still favor prolonged bed rest in this patient population.66–69
Direct pressure at the durotomy site with a pressure dressing or brace may counteract egress through the area of least resistance.39 We argue that it is reasonable to try Trendelenburg positioning and direct pressure at the site of durotomy for a short course of 1 to 3 days in someone with a suspected lumbar durotomy. The role of antibiotic therapy for CSF fistulas and pseudomeningoceles is not clear. However, an analysis of published reports suggested that antibiotics do not decrease the short- or long-term incidence of meningitis among patients with cranial CSF fistulas or pseudomeningoceles.70
If symptoms continue or worsen, as in this case, escalation of care to non-conservative therapy should begin. These non-conservative options include epidural blood patch, CSF diversion measures, and reoperation for primary closure attempt or reattempt. In this case, it is unclear why the surgeon waited until POD 25 to readmit the patient, and even then, did not reimage the patient, apparently satisfied by not finding a visible dural defect during wound exploration. Several previous PSNet cases have illustrated how providers may fail to escalate treatment for post-procedural complications in a timely manner when they lack knowledge or experience, have ineffective communication or diffusion of responsibility among team members, or “anchor” on a single diagnosis or treatment strategy despite evolving evidence of its failure.
Non-Conservative Approach
Epidural Blood Patch
Epidural blood patch (EBP) is a procedure in which the patient’s own blood is injected into the epidural space. It is thought that the blood forms a clot over the dural tear, allowing healing of the dura. The EBP also raises extradural pressure relative to the subarachnoid pressure to decrease the efflux of CSF.71
CSF Diversion
A large pseudomeningocele is best treated by a combination of primary closure and implantation of a subarachnoid drain.72 These drains are efficacious at an output rate of 5-10 mL/hr for 3-5 days, as a primary tool or supplement to other measures.73 However, lumbar subarachnoid drainage has been reported to have a complication rate of up to 44% with symptoms ranging from headache and nerve root pain to subdural hematoma, pneumocephalus, and meningitis.74 These symptoms are important to consider when choosing to use a lumbar subarachnoid drain.
Lastly, there have been reports of a ventriculoperitoneal or lumboperitoneal shunt being used successfully to treat a pseudomeningocele and CSF fistula/leak.75–78 However, this approach should only be considered as a last resort, after an attempt/reattempt at primary closure has failed.
Approaches to Improving Patient Safety
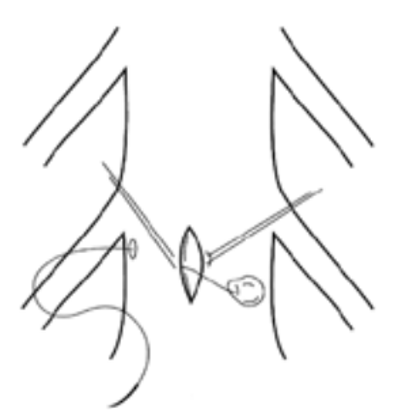
Avoidance of a durotomy, if not in the plan of the operation, is critical. The necessary dissections should precede each Kerrison rongeur bite during a laminectomy to ensure that the dura mater does not come between the footplate of the rongeur and bone. The movement of the drill during a laminectomy should be directed laterally so that even with a slip, a dural tear may be avoided. An instrument should cover the exposed dura during the drilling. When a dural violation is appreciated, primary closure should be attempted to prevent future complications. The goal is “watertight” closure of the dura.33 Extending the laminectomy may be necessary to gain better exposure to repair the dura, while using magnification via loupes or a microscope. For durotomies that occur in inaccessible areas, such as far-lateral durotomies, a small plug of muscle or fat may be introduced through a more medial, intentional durotomy and pulled into the defect (Figure 5).8
Figure 5. A Schematic Drawing of Mayfield’s Midline Durotomy in Which a Far-Lateral Dural Tear is Repaired
If a patient with a suspected lumbar durotomy is exhibiting symptoms, it is reasonable to try Trendelenburg positioning for a short course of 1 to 3 days. Direct pressure at the site of durotomy with a pressure dressing or brace may counteract egress through the area of least resistance.39 Kim et al published a case report where a polycarbonate face mask was used to apply pressure on a refractory pseudomeningocele,39 leading to resolution. One should suspect a CSF leak if symptoms persist; imaging and laboratory testing should be considered for confirmation. If a durotomy went unnoticed intraoperatively, or a “watertight” closure could not be obtained, and the patient remains symptomatic after several conservative therapies have been employed, then timely escalation to non-conservative therapy is essential. Failure to do so in this case suggests implicit bias, perhaps due to the patient's age, cultural or communication barriers, or perceived lack of adherence to bed rest. Non-conservative therapies include epidural blood patch and lumbar subarachnoid drain placement. If these measures continue to fail, reoperation to obtain a primary closure should be attempted or reattempted, and in rare cases, placement of a ventriculo/lumboperitoneal shunt should be considered.
Take-Home Points
- The best offense is a good defense and avoidance of a durotomy, if not in the plan of the surgery, is key to preventing complications.
- If a dural violation occurs, the primary goal should be achieving a “watertight” closure. This can be done using patches, grafts, and sealants to reinforce the primary repair.
- In cases where a watertight closure is not achieved, or if a durotomy goes unnoticed and the patient develops symptoms, immediate management of the CSF leak is crucial.
- Management of a CSF leak should begin conservatively, with bed rest and local pressure dressings. If these measures fail, escalate care promptly to include interventions such as an epidural blood patch or lumbar subarachnoid drain placement. Prolonged bed rest at home is not appropriate.
- If all measures continue to fail, a reoperation to achieve primary closure is needed to avoid severe complications. In rare cases, the placement of a ventriculoperitoneal or lumboperitoneal shunt may be considered.
Jose A Castillo Jr, MD
Department of Neurological Surgery
UC Davis Health
jcastillo@ucdavis.edu
Richard Price, MD, PhD
Assistant Professor
Department of Neurological Surgery
UC Davis Health
riprice@ucdavis.edu
Kee D Kim, MD
Professor
Chief, Spinal Neurosurgery
Co-Director, UC Davis Spine Center
UC Davis Health
kdkim@ucdavis.edu
Editor's Note: this case was adapted from Capozzola DD, Terrence J. Mixed Defense Ruling Related to Patient's Death Yield Lessons Regarding Experts. Healthcare Risk Management (Relias Media). Published March 1, 2024.
References
- Miller PR, Elder FW. Meningeal pseudocysts (meningocele spurius) following laminectomy. Report of ten cases. J Bone Joint Surg Am. 1968;50(2):268-276. [Available at]
- Pagni CA, Cassinari V, Bernasconi V. Meningocele spurius following hemilaminectomy in a case of lumbar discal hernia. J Neurosurg. 1961;18:709-710.[ Available at]
- Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2000;9(1):e5. [Free full text]
- Jones AA, Stambough JL, Balderston RA, et al. Long-term results of lumbar spine surgery complicated by unintended incidental durotomy. Spine (Phila Pa 1976). 1989;14(4):443-446. [Available at]
- Lee KS, Hardy IM. Postlaminectomy lumbar pseudomeningocele: report of four cases. Neurosurgery. 1992;30(1):111-114. [Available at]
- O’Connor D, Maskery N, Griffiths WE. Pseudomeningocele nerve root entrapment after lumbar discectomy. Spine (Phila Pa 1976). 1998;23(13):1501-1502.[ Available at]
- Oppel F, Schramm J, Schirmer M, et al. Results and Complicated Course after Surgery for Lumbar Disc Herniation. In: Wüllenweber R, Brock M, Hamer J, Klinger M, Spoerri O, eds. Lumbar Disc Adult Hydrocephalus. Vol 4. Advances in Neurosurgery. Springer Berlin Heidelberg; 1977:36-51. [Available at]
- Mayfield FH, Kurokawa K. Watertight closure of spinal dura mater. Technical note. J Neurosurg. 1975;43(5):639-640. [Available at]
- Rodriguez-Olaverri JC, Zimick NC, Merola A, et al. Comparing the clinical and radiological outcomes of pedicular transvertebral screw fixation of the lumbosacral spine in spondylolisthesis versus unilateral transforaminal lumbar interbody fusion (TLIF) with posterior fixation using anterior cages. Spine (Phila Pa 1976). 2008;33(18):1977-1981.[ Available at]
- David K, Rao R, Fischgrund J. Postoperative Pseudomeningocele, Hematoma, and Seroma. Musculoskeletal Key. 2016. Accessed November 6, 2024. [Free full text]
- Tsuji H, Handa N, Handa O, et al. Postlaminectomy ossified extradural pseudocyst. Case report. J Neurosurg. 1990;73(5):785-787. [Available at]
- Bhatoe HS. Postoperative lumbar pseudomeningocele. J Indian Med Assoc. 1995;93(7):280, 282. [PubMed citation]
- Goodman SJ, Gregorius FK. Cervical pseudomeningocele after laminectomy as a cause of progressive myelopathy. Bull Los Angeles Neurol Soc. 1974;39(3):121-127. [PubMed citation]
- Mammadkhanli O, Elbir C, Hanalioglu S, et al. Subfascial drainage and clipping technique for treatment of cerebrospinal fluid leak following spinal surgery. Neurosciences (Riyadh). 2020;25(1):50-54. [Free full text]
- Couture D, Branch CL. Spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2003;15(6):E6. [Free full text]
- Rocca A, Turtas S, Pirisi A, et al. Iatrogenic lumbar pseudomeningocele. Zentralbl Neurochir. 1986;47(4):311-315. [Available at]
- Aldrete JA, Ghaly R. Postlaminectomy pseudomeningocele. An unsuspected cause of low back pain. Reg Anesth. 1995;20(1):75-79. [Available at]
- Huff T, Tadi P, Weisbrod LJ, et al. Neuroanatomy, Cerebrospinal Fluid. In: StatPearls. StatPearls Publishing; 2024. Accessed July 20, 2024. [Free full text]
- Cobb C, Ehni G. Herniation of the spinal cord into an iatrogenic meningocele. Case report. J Neurosurg. 1973;39(4):533-536. [Available at]
- Maiuri F, Corriero G, Giamundo A, et al. Postoperative cervical pseudomeningocele. Neurochirurgia (Stuttg). 1988;31(1):29-31. [Available at]
- Burres KP, Conley FK. Progressive neurological dysfunction secondary to postoperative cervical pseudomeningocele in a C-4 quadriplegic. Case report. J Neurosurg. 1978;48(2):289-291. [Available at]
- Hadani M, Findler G, Knoler N, et al. Entrapped lumbar nerve root in pseudomeningocele after laminectomy: report of three cases. Neurosurgery. 1986;19(3):405-407. [Available at]
- Helle T, Conley FK. Postoperative Cervical pseudomeningocele as a cause of delayed myelopathy. Neurosurgery. 1981;9(3):314-316. [Available at]
- Horowitz SW, Azar-Kia B, Fine M. Postoperative cervical pseudomeningocele. AJNR Am J Neuroradiol. 1990;11(4):784. [Free full text]
- Hosono N, Yonenobu K, Ono K. Postoperative cervical pseudomeningocele with herniation of the spinal cord. Spine (Phila Pa 1976). 1995;20(19):2147-2150. [Available at]
- Rinaldi I, Hodges TO. Iatrogenic lumbar meningocoele: report of three cases. J Neurol Neurosurg Psychiatry. 1970;33(4):484-492. [Free full text]
- Wilkinson HA. Nerve-root entrapment in “traumatic” extradural arachnoid cyst. J Bone Joint Surg Am. 1971;53(1):163-166. [Available at]
- Bell WE, Joynt RJ, Sahs AL. Low spinal fluid pressure syndromes. Neurology. 1960;10:512-521. [Available at]
- Hochman MS, Naidich TP, Kobetz SA, et al. Spontaneous intracranial hypotension with pachymeningeal enhancement on MRI. Neurology. 1992;42(8):1628-1630. [Available at]
- Schumacher HW, Wassmann H, Podlinski C. Pseudomeningocele of the lumbar spine. Surg Neurol. 1988;29(1):77-78. [Available at]
- Ryall RG, Peacock MK, Simpson DA. Usefulness of beta 2-transferrin assay in the detection of cerebrospinal fluid leaks following head injury. J Neurosurg. 1992;77(5):737-739. [Available at]
- Normansell DE, Stacy EK, Booker CF, Butler TZ. Detection of beta-2 transferrin in otorrhea and rhinorrhea in a routine clinical laboratory setting. Clin Diagn Lab Immunol. 1994;1(1):68-70. [Free full text]
- Barth M, Tuettenberg J, Thomé C, Weiss C, Vajkoczy P, Schmiedek P. Watertight dural closure: is it necessary? A prospective randomized trial in patients with supratentorial craniotomies. Neurosurgery. 2008;63(4 Suppl 2):352-358; discussion 358. [Available at]
- Hannallah D, Lee J, Khan M, Donaldson WF, Kang JD. Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am. 2008;90(5):1101-1105. [Available at]
- Kitchel SH, Eismont FJ, Green BA. Closed subarachnoid drainage for management of cerebrospinal fluid leakage after an operation on the spine. J Bone Joint Surg Am. 1989;71(7):984-987. [Available at]
- Eismont FJ, Wiesel SW, Rothman RH. Treatment of dural tears associated with spinal surgery. J Bone Joint Surg Am. 1981;63(7):1132-1136. [Available at]
- Foyt D, Johnson JP, Kirsch AJ, Bruce JN, Wazen JJ. Dural closure with laser tissue welding. Otolaryngol Head Neck Surg. 1996;115(6):513-518. [Available at]
- Levy DI, Sonntag VK. Titanium dural clip testing. Technical note. J Neurosurg. 1994;81(6):947-949. [Available at]
- Kerr E, Ripul P, Kim KD. Refractory Cervical Spinal Pseudomeningocele Successfully Treated with a Polycarbonate Face Mask: Technical Note. JSM Neurosurgery and Spine. 2014;2(1):1011. [Free full text]
- Shimada Y, Hongo M, Miyakoshi N, et al. Dural substitute with polyglycolic acid mesh and fibrin glue for dural repair: technical note and preliminary results. J Orthop Sci. 2006;11(5):454-458. ]Free full text]
- Chu CC, Pratt L, Zhang L, Hsu A, Chu A. A comparison of a new polypropylene suture with Prolene. J Appl Biomater. 1993;4(2):169-181. [Available at]
- Megyesi JF, Ranger A, MacDonald W, Del Maestro RF. Suturing technique and the integrity of dural closures: an in vitro study. Neurosurgery. 2004;55(4):950-954; discussion 954-955. [Available at]
- Ebel F, Wanderer S, Beck J, Raabe A, Ulrich CT. The Optimal Dura Closure Technique and Material – An In-Vitro Evaluation. J Neurol Surg A Cent Eur Neurosurg. 2017;78(S 01):S1-S22. [Available at]
- Ghobrial GM, Maulucci CM, Viereck MJ, et al. Suture Choice in Lumbar Dural Closure Contributes to Variation in Leak Pressures: Experimental Model. Clin Spine Surg. 2017;30(6):272-275. [Available at]
- Bakhsheshian J, Strickland BA, Patel NN, et al. The use of a novel perfusion-based cadaveric simulation model with cerebrospinal fluid reconstitution comparing dural repair techniques: a pilot study. Spine J. 2017;17(9):1335-1341. [Available at]
- Kelz RR, Sellers MM, Niknam BA, et al. A National Comparison of Operative Outcomes of New and Experienced Surgeons. Ann Surg. 2021;273(2):280-288. [Free full text]
- Kumar CM, Van Zundert AAJ. Intraoperative Valsalva maneuver: a narrative review. Can J Anaesth. 2018;65(5):578-585. [Free full text]
- MacEwan MR, Kovacs T, Osbun J, Ray WZ. Comparative analysis of a fully-synthetic nanofabricated dura substitute and bovine collagen dura substitute in a large animal model of dural repair. Interdisciplinary Neurosurgery. 2018;13:145-150. [Free full text]
- Integra. Accessed November 6, 2024. [Available at]
- Haq I, Cruz-Almeida Y, Siqueira EB, et al. Postoperative fibrosis after surgical treatment of the porcine spinal cord: a comparison of dural substitutes. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2005;2(1):50-54. [Available at]
- Integra. Integra Promotional Material #0069447- 3-EN. Accessed November 6, 2024. [Free full text]
- Epstein NE, Hollingsworth R. Anterior cervical micro-dural repair of cerebrospinal fluid fistula after surgery for ossification of the posterior longitudinal ligament. Technical note. Surg Neurol. 1999;52(5):511-514. [Free full text]
- Cain JE, Rosenthal HG, Broom MJ, et al. Quantification of leakage pressures after durotomy repairs in the canine. Spine (Phila Pa 1976). 1990;15(9):969-970. [Available at]
- Pomeranz S, Constantini S, Umansky F. The use of fibrin sealant in cerebrospinal fluid leakage. Neurochirurgia (Stuttg). 1991;34(6):166-169.[ Free full text]
- Tisseel. Baxter Advanced Surgery. Accessed November 6, 2024. [Free full text]
- Mulder M, Crosier J, Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: case report. Spine (Phila Pa 1976). 2009;34(4):E144-E148. [Available at]
- Tseng WL, Xiao F. Duraseal thecal sac compression after lumbar discectomy causing radiculopathy. Spine J. 2015;15(8):1892-1893. [Available at]
- Wright NM, Park J, Tew JM, et al. Spinal sealant system provides better intraoperative watertight closure than standard of care during spinal surgery: a prospective, multicenter, randomized controlled study. Spine (Phila Pa 1976). 2015;40(8):505-513. [Available at]
- Kim KD, Ramanathan D, Highsmith J, et al. DuraSeal Exact Is a safe adjunctive treatment for durotomy in spine: postapproval study. Global Spine J. 2019;9(3):272-278. [Free full text]
- FDA. Premarket Approval (PMA). US Food & Drug Administration. Accessed November 6, 2024. [Free full text]
- Fang Z, Tian R, Jia YT, et al. Treatment of cerebrospinal fluid leak after spine surgery. Chin J Traumatol. 2017;20(2):81-83. [Free full text]
- Hodges SD, Humphreys SC, Eck JC, et al. Management of incidental durotomy without mandatory bed rest. A retrospective review of 20 cases. Spine (Phila Pa 1976). 1999;24(19):2062-2064. [Available at]
- Khan MH, Rihn J, Steele G, et al. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: a review of 3,183 consecutive degenerative lumbar cases. Spine (Phila Pa 1976). 2006;31(22):2609-2613. [Available at]
- Winter F, Hasslinger S, Frueh A, et al. Incidence, risk factors, and treatment of incidental durotomy during decompression in degenerative lumbar spine conditions. J Neurosurg Sci. 2023;67(4):507-511. [Available at]
- Gomes FC, Larcipretti ALL, Elvir FAR, et al. Early ambulation versus prolonged bed rest for incidental durotomies in spine procedures: a systematic review and meta-analysis. Neurosurg Rev. 2023;46(1):310. [Free full text]
- Oitment C, Aref M, Almenawar S, et al. Spinal Dural Repair: A Canadian Questionnaire. Global Spine Journal. 2018;8(4):359-364. [Free full text]
- Clajus C, Stockhammer F, Rohde V. The intra- and postoperative management of accidental durotomy in lumbar spine surgery: results of a German survey. Acta Neurochir (Wien). 2015;157(3):525-530. [Available at]
- Lewandrowski KU, Hellinger S, De Carvalho PST, et al. Dural tears during lumbar spinal endoscopy: surgeon skill, training, incidence, risk factors, and management. Int J Spine Surg. 2021;15(2):280-294. [Free full text]
- Gautschi OP, Stienen MN, Smoll NR, et al. Incidental durotomy in lumbar spine surgery--a three-nation survey to evaluate its management. Acta Neurochir (Wien). 2014;156(9):1813-1820. [Available at]
- Eljamel MS. Antibiotic prophylaxis in unrepaired CSF fistulae. Br J Neurosurg. 1993;7(5):501-505. [Available at]
- McCormack BM, Taylor SL, Heath S, et al. Pseudomeningocele/CSF fistula in a patient with lumbar spinal implants treated with epidural blood patch and a brief course of closed subarachnoid drainage. A case report. Spine (Phila Pa 1976). 1996;21(19):2273-2276. [Available at]
- Weng YJ, Cheng CC, Li YY, et al. Management of giant pseudomeningoceles after spinal surgery. BMC Musculoskelet Disord. 2010;11:53. [Free full text]
- Shapiro SA, Scully T. Closed continuous drainage of cerebrospinal fluid via a lumbar subarachnoid catheter for treatment or prevention of cranial/spinal cerebrospinal fluid fistula. Neurosurgery. 1992;30(2):241-245. [Available at]
- Açikbaş SC, Akyüz M, Kazan S, et al. Complications of closed continuous lumbar drainage of cerebrospinal fluid. Acta Neurochir (Wien). 2002;144(5):475-480. Available at
- Duthel R, Nuti C, Motuo-Fotso MJ, et al. [Complications of lumboperitoneal shunts. A retrospective study of a series of 195 patients (214 procedures)]. Neurochirurgie. 1996;42(2):83-89; discussion 89-90. [PubMed citation]
- James HE, Tibbs PA. Diverse clinical applications of percutaneous lumboperitoneal shunts. Neurosurgery. 1981;8(1):39-42. [Available at]
- Katz SS, Savitz MH, Osei C, et al. Successful treatment by lumboperitoneal shunting of a spinal subclavicular fistula following thoracotomy. Neurosurgery. 1982;11(6):795-796. [Available at]
- Kitchen N, Bradford R, Platts A. Occult spinal pseudomeningocele following a trivial injury successfully treated with a lumboperitoneal shunt: a case report. Surg Neurol. 1992;38(1):46-49. [Available at]



