The Lung Nodule That Refused To Grow
Balekian AA, Gould MK. The Lung Nodule That Refused To Grow. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2012.
Balekian AA, Gould MK. The Lung Nodule That Refused To Grow. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2012.
Case Objectives
- Define a solitary pulmonary nodule.
- Identify the different initial first steps of management.
- Identify factors associated with malignant or benign disease.
- Understand when patients no longer need surveillance for benign disease.
Case & Commentary—Part 1:
After moving to a new city, a 67-year-old man presented to a primary care physician for an initial visit to establish care. In discussing his past medical history, the patient described having a "spot" on his lungs that doctors had been following since 2004.
The solitary pulmonary nodule (SPN) can be defined as a single, well-circumscribed radiographic density measuring less than 3 cm, surrounded by aerated lung, and without any evidence of atelectasis, hilar enlargement, or pleural effusion.(1) Depending on the study population, between 15% and 75% of these nodules prove to be malignant.(2,3) The differential diagnosis includes malignant, infectious, inflammatory, and miscellaneous benign etiologies (Table 1).
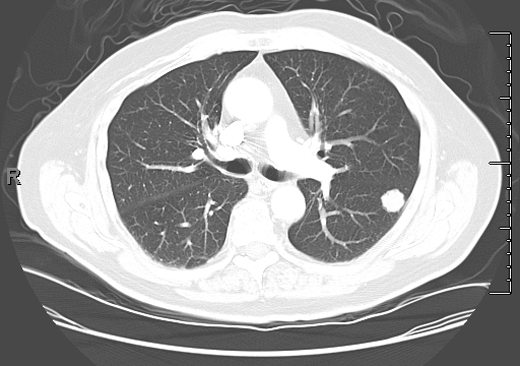
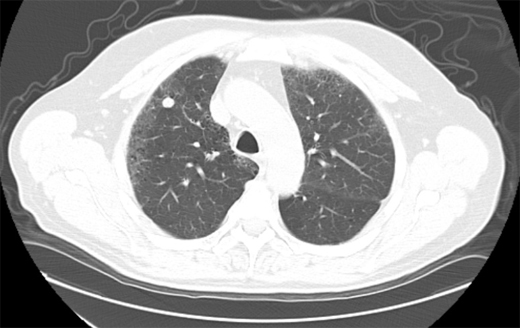
In the past, most nodules were discovered incidentally on plain chest radiographs, as in this case. In recent years, it is more common for nodules to be identified by chest computed tomography (CT). However, nodules detected by CT often do not meet the classic definition of the SPN because either more than one nodule or an accompanying finding like atelectasis is present. Except for obviously calcified nodules (which can be attributed to old granulomatous disease), non-calcified nodules seen on chest radiography should be confirmed and better characterized by chest CT. CT characteristics associated with a benign etiology include smaller size, fat density, or a central, diffuse, or popcorn pattern of calcification. CT characteristics associated with malignancy include larger size, upper lobe location, irregular margins or spiculation, thick-walled cavitation, and hilar or mediastinal lymphadenopathy (Figures 1–3).
Patient-specific risk factors for malignancy include older age, current or former smoking status, prior history of extrathoracic malignancy, and asbestos exposure. Although most clinicians intuitively estimate the probability of malignancy, validated clinical prediction models can help refine decision-making. The Memorial Sloan-Kettering model calculates a patient's 10-year risk of developing lung cancer; although this model estimates risk before any radiographic imaging has been performed, it might be helpful in identifying patients in whom CT screening for lung cancer should be discussed.(4) Two other models—the Mayo Clinic model and the Veterans Affairs model—combine patient-level and radiographic characteristics to estimate the probability of malignancy for patients in whom a nodule has already been identified.(5,6) Based on a patient's probability for malignancy (low, intermediate, high), clinicians can use these models to choose an appropriate management strategy: monitoring with serial CT scans at designated intervals, performing functional imaging (e.g., positron emission tomography [PET]) to characterize the nodule further, or proceeding directly to biopsy (or even surgical resection) without further testing. The Mayo Clinic model has been validated in other populations and is widely used in practice to distinguish between nodules with a low risk of cancer (which can often be followed radiographically) and those with a high risk of cancer (which require tissue diagnosis and/or prompt resection).(7,8)
The most common avoidable error in the management of lung nodules is neglecting to review prior imaging studies. This can be essential in management; for example, if a solid nodule has been present for 2 or more years and has not changed, this is very strong presumptive evidence of a benign etiology, and no additional follow-up is required.(9) For sub-solid (ground glass) nodules, which are frequently premalignant or malignant but slow-growing, it may be necessary to demonstrate longer periods of stability to exclude malignancy.
The next most common error is to choose a strategy of "wait and watch" but neglect to "watch." This error ranges in severity from a minor delay in imaging (such as missing a single examination) to its most severe manifestation, in which the patient is lost to all follow-up either due to miscommunication or lapses in adherence. To date, robust electronic reminder systems for nodule evaluation have not been widely adopted, but there is a clear need for developing and testing these kinds of system-level solutions. Fortunately, the surveillance strategy is typically selected when the probability of cancer is very low or low, so this type of error infrequently results in harm.
Some might argue that it is an error to choose surveillance (as opposed to immediate intervention) for a nodule that ultimately proves to be cancerous. However, as long as this choice was the product of thoughtful deliberation that considered the risk of cancer and the benefits and harms of competing alternatives, we would characterize this as an undesirable outcome that resulted from an appropriate decision. Of note, there have been few systematic studies of outcomes following delayed diagnosis in patients with cancerous nodules, so the magnitude of harm associated with delay is highly uncertain.
Analogously, surgical resection of a benign nodule, although undesirable, may represent an appropriate course of action for a nodule that was considered likely to be malignant. In lung nodule evaluation, as in other areas of medicine, a bad outcome does not necessarily mean that an error was made.
However, it may be an error in judgment to select surveillance for a nodule that is likely to be cancerous, or to select surgical diagnosis for a nodule that is likely to be benign, highlighting the importance of accurately estimating the clinical probability of cancer. Such discordance should be infrequent, except for cases in which there are extenuating circumstances (e.g., strong patient preferences).
Case & Commentary—Part 2:
Upon further history, the patient stated that while undergoing hernia surgery in 2004, a nodule was seen on a routine chest radiograph. This had been followed up at the time with a CT scan and a PET scan. Based on these results, the nodule was felt to be benign and not an active malignancy or infection. The patient had no symptoms from the nodule.
After chest CT has been performed, there are four possible alternatives for the next step in nodule evaluation: PET scan, non-surgical biopsy, surgical diagnosis, or surveillance.
PET scans use 18-fluorodeoxyglucose (18-FDG) to measure the metabolic activity of a nodule. Although malignant cells are more metabolically active (and will therefore "light up" on a PET scan), infections and other types of inflammation (e.g., granulomatous) will also yield positive results, thereby compromising the specificity of the test. Smaller malignant nodules measuring less than 1 cm and sub-solid (ground glass) nodules can be missed by a PET scan.(10) The American College of Chest Physicians (ACCP) recommends PET for characterization of solid nodules measuring larger than 8 mm with a low to moderate probability for malignancy.(11) If PET shows increased uptake, this would prompt either biopsy or prompt resection. If PET shows no increased uptake, then watchful waiting with periodic surveillance is usually the preferred course.
Non-surgical biopsy is less invasive than surgical diagnosis and can be performed via transthoracic needle biopsy (TTNB) for peripheral lesions or bronchoscopy for central lesions. Endobronchial ultrasound (EBUS) and electromagnetic-guided navigational bronchoscopy (a computer program–aided navigational system for localizing lung nodules) increase the yield of bronchoscopic fine needle aspiration over routine bronchoscopy.(12) In addition, bronchoscopic procedures can simultaneously stage the mediastinal or hilar lymph nodes in the case of lung cancer.(13) For most nodules, CT-guided TTNB will have a higher yield than the newer guided bronchoscopic techniques, but it carries a higher risk of pneumothorax requiring chest tube placement (~7%).(14) At this point, the choice between these techniques will largely be based on the judgment and experience of the involved physicians, along with patient preference.
Surgical diagnosis, usually via a video-assisted thorascopic surgery (VATS), is often preferred for nodules that are very likely to be cancerous. The risk of a fatal complication from VATS wedge resection is low (
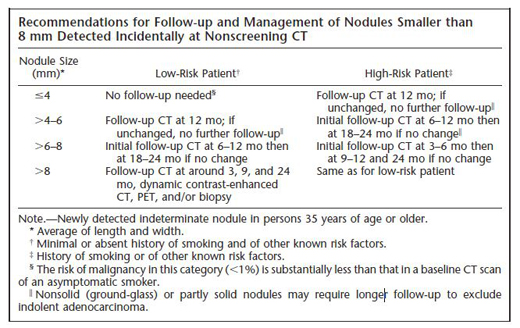
In the case under discussion, the nodule was "felt to be benign" based on the CT and PET results, suggesting that there was little or no FDG uptake on the PET scan. While this is reassuring, even a negative PET scan does not necessarily exclude the possibility of malignancy and additional follow-up is required, especially for sub-solid (ground glass) nodules. The optimal duration and frequency of surveillance is uncertain. For small (≤8 mm) solid nodules, most radiologists and pulmonologists defer to the expert consensus-based recommendations of the Fleischner Society (Table 2).(15) Although their recommendations are reasonable, it is important to note that they have not been prospectively validated. For larger (>8 mm) solid nodules, the ACCP recommends 3 rounds of surveillance (using non-contrast, low-dose techniques) at 3–6, 9–12, and 18–24 months.(11) For sub-solid (ground glass) nodules, a consensus is emerging that follow-up longer than 2 years is required to exclude slowly growing cancers, but the risks of extended duration surveillance (radiation exposure, workup of harmless incidental findings) should be weighed in relation to the putative benefits.
Case & Commentary—Part 3:
The patient had a 6-month follow-up CT scan in 2004 confirming the presence of the nodule and that it had not grown or changed in character. Subsequently, the patient had CT scans of the chest with and without contrast every 6–12 months for 8 years that showed no change in the size or character of the nodule. In total, he had more than 20 CT scans of the chest. The new primary care doctor wondered if all of the CT scans were necessary and how the nodule should have been managed.
Although the details of this case are murky, 8 years of follow-up is unquestionably excessive. Even for a sub-solid nodule, there is no evidence that follow-up of this duration is beneficial. Sub-solid nodules are usually identified by chest CT, and most are probably missed by chest radiography, so the nodule in this case was likely solid in appearance and perhaps even calcified. If benign calcification had been identified on the chest CT scan, then no additional follow-up would have been required. If the nodule was solid but non-calcified, as we suspect, then 2 years of radiographic stability is usually sufficient to exclude malignancy. While the harm caused by these additional CT scans is arguable, some studies have estimated that as few as 1 in 3000 patients scanned or as many as 1 in 300 patients scanned will develop cancer, depending on their age and sex.(16,17) Of course, there is also economic harm, with each scan costing several hundred, and in some settings, several thousand, dollars.
Take-Home Points
- Small pulmonary nodules (≤8 mm) are usually managed by CT surveillance.
- For small solid nodules, the frequency and duration of surveillance are guided by recommendations from the Fleischner Society, which vary depending on the size of the nodule and the presence or absence of risk factors for cancer (Table 2).
- For sub-solid nodules, which often represent low-grade cancers, the optimal frequency and duration of follow-up are highly uncertain, although there is an emerging consensus favoring extended duration follow-up in the range of 3 to 5 years.
- A systematic approach to evaluating larger (>8 mm), solid nodules includes the following steps:
- Review old imaging studies.
- Estimate the clinical probability of malignancy by considering both patient risk factors and nodule characteristics, or by using a validated prediction model.
- Use functional imaging (typically with FDG-PET) to further characterize nodules when the clinical probability is low to moderate.
- Provide information about the likelihood of cancer, the risks of procedures, and the potential benefits and harms associated with each of the alternatives for evaluation. Tailor the information about risk to the specific patient, when possible.
- Elicit patient preferences for alternatives and outcomes (e.g., unnecessary surgery for a benign nodule versus delayed diagnosis and treatment of a malignant nodule). Help the patient make the best choice between CT surveillance, non-surgical biopsy, and surgical diagnosis.
Alex A. Balekian, MD, MSHS
Assistant Professor of Clinical Medicine
Keck School of Medicine of USC
Michael K. Gould, MD, MS
Senior Research Scientist and Assistant Director for Health Services Research
Department of Research and Evaluation
Kaiser Permanente Southern California
Faculty Disclosure: Dr. Balekian and Dr. Gould have declared that neither they, nor any immediate member of their families, have a financial arrangement or other relationship with the manufacturers of any commercial products discussed in this continuing medical education activity. In addition, the commentary does not include information regarding investigational or off-label use of pharmaceutical products or medical devices. Dr. Balekian is supported by a KL2 research training award from the Southern California Clinical Translational Science Institute (KL2 RR31991). Dr. Gould receives grant support from NIH/NCI to evaluate clinical decision support for lung nodule management (R01 CA117840).
References
1. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med. 2003;348:2535-2542. [go to PubMed]
2. Khouri NF, Meziane MA, Zerhouni EA, Fishman EK, Siegelman SS. The solitary pulmonary nodule. Assessment, diagnosis, and management. Chest. 1987;91:128-133. [go to PubMed]
3. Swensen SJ, Morin RL, Schueler BA, et al. Solitary pulmonary nodule: CT evaluation of enhancement with iodinated contrast material—a preliminary report. Radiology. 1992;182:343-347. [go to PubMed]
4. Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470-478. [go to PubMed]
5. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849-855. [go to PubMed]
6. Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131:383-388. [go to PubMed]
7. Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128:2490-2496. [go to PubMed]
8. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63:335-341. [go to PubMed]
9. Fein AM, Feinsilver SH, Ares CA. The solitary pulmonary nodule: a systemic approach. In: Fishman AP, ed. Fishman's Pulmonary Diseases and Disorders. 3rd ed. Vol. 2. New York, NY: McGraw-Hill; 1998. ISBN: 9780079111678.
10. Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914-924. [go to PubMed]
11. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer?: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143(suppl 5):e93S-e120S. [go to PubMed]
12. Eberhardt R, Anantham D, Herth F, Feller-Kopman D, Ernst A. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest. 2007;131:1800-1805. [go to PubMed]
13. Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc. 2009;6:180-186. [go to PubMed]
14. Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155:137-144. [go to PubMed]
15. MacMahon H, Austin JHM, Gamsu, G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395-400. [go to PubMed]
16. Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078-2086. [go to PubMed]
17. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077. [go to PubMed]
Tables
Table 1. Differential Diagnosis of a Solitary Pulmonary Nodule
| Malignant | Primary lung cancer, metastatic cancer, pulmonary carcinoid tumor |
| Infectious | Coccidiomycosis, blastomycosis, histoplasmosis, crypotococcus, lung abscess, tuberculosis, dirofilariasis |
| Rheumatologic | Rheumatoid nodule, granulomatosis with polyangiitis |
| Miscellaneous benign causes | Hamartoma, healed granuloma, pseudonodule (loculated pleural fluid), pulmonary infarct, pulmonary arteriovenous malformation |
Table 2. Fleischner Society recommendations for follow-up of small solid pulmonary nodules. Reprinted from (15) with permission from Radiological Society of North America.
Figures
Figure 1. Solid nodule with irregular border. Biopsied immediately. Diagnosis: lung cancer.
Figure 2. Solid nodule with eccentric calcification. Followed over 2 years. Diagnosis: Stable nodule.
Figure 3. Solid nodule with lobulated border. Followed over 2 years. Diagnosis: Stable nodule.