Coming Undone: Failure of Closure Device
Baez-Escudero JL, Levine GN. Coming Undone: Failure of Closure Device. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2007.
Baez-Escudero JL, Levine GN. Coming Undone: Failure of Closure Device. PSNet [internet]. Rockville (MD): Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2007.
The Case
A 65-year-old man underwent coronary angiography because of atypical exertional chest pain and shortness of breath. He was found to have coronary artery disease with significant narrowing of the proximal left anterior descending artery and a number of narrowings more distally. A bare metal stent was placed in the proximal lesion. A number of attempts were made to place more distal stents, but they could not be positioned correctly. After the procedure, the femoral artery sheath was removed and hemostasis was achieved with the use of an angio-seal closure device. A cardiovascular surgeon was consulted that day, and bypass surgery was scheduled for 4 days later.
The day after angiography, the patient, who was now home, was active and playing with his visiting grandchildren. The next day, the patient developed bleeding from the catheter site in his groin. The bleeding was not stopped by local pressure, and he returned to the hospital, where he was found to be tachycardic and hypotensive. His hematocrit dropped from 42% to 36%, and a computed tomography (CT) scan revealed a large (14 cm) retroperitoneal hematoma. He was taken to the operating room, and it was discovered the angio-seal closure device had failed and the femoral artery puncture (arteriotomy) was repaired.
The patient subsequently had complications related to the retroperitoneal hematoma, including persistent fever, leukocytosis, ileus, and back pain. His coronary artery bypass surgery was delayed, and while hospitalized waiting for the procedure, he suffered a cardiac arrest and died.
The Commentary
Cardiac catheterization is a diagnostic procedure that can be used to evaluate cardiac hemodynamics, ventricular function, and coronary artery anatomy. The introduction of percutaneous coronary intervention (PCI), including balloon angioplasty and stenting, as a therapeutic adjunct to catheterization has revolutionized the contemporary treatment of cardiovascular disease. In 2004, the estimated number of inpatient diagnostic catheterizations was 1,471,000, and the number of inpatient PCI procedures was 1,285,000.(1) Adding outpatient diagnostic and therapeutic catheterizations would significantly increase these numbers.
Diagnostic catheterization and PCI are associated with a variety of adverse events, ranging from minor problems without long-term sequelae to major complications requiring immediate therapy. This case of a 65-year-old man—who underwent coronary angiography and had a major complication that required an unplanned vascular surgical repair, delayed his necessary bypass surgery, and ultimately led to his death—illustrates one of the complications of cardiac catheterization and PCI. In the following discussion, the most common complications of cardiac catheterization and measures to prevent, diagnose, and treat these complications are reviewed.
The risk of a major complication (those considered serious or life-threatening) during diagnostic cardiac catheterization is less than 1%–2%. In one large series, the multivariate predictors of major complications were cardiogenic shock, acute myocardial infarction (MI), renal insufficiency, cardiomyopathy, valvular disease, poorly compensated heart failure, severe hypertension, and unstable angina.(2)
The rate of catheterization-related death has declined steadily over the past 15 years and is now approximately 0.08%.(3) The risk of death varies with age, as well as with the presence of the clinical predictors mentioned above. Patients with severe narrowing of the left main coronary artery and poor left ventricular function (ejection fraction [EF] 2-4)
Embolization of thrombus, air, or atheromatous debris is an important cause of periprocedural stroke and MI. The rate of procedure-related stroke, one of the most devastating complications of cardiac catheterization, was as high as 0.23% in 1973 (3) but has decreased to 0.06% in contemporary registries.(5) The current risk rate for procedure-related MI is less than 0.03%. Risk factors for suffering an acute MI during the procedure include recent unstable angina or non–Q-wave infarction, severe coronary artery disease, and the presence of important comorbidities.(2-4)
Administration of radiocontrast agents used during catheterization may cause complications such as allergic reactions or renal failure. Reactions to the iodinated contrast agents commonly used occur in approximately 1% of patients.(6) These result from direct complement activation and are classified as anaphylactoid reactions. Symptoms include urticaria, angioedema, bronchospasm, and hypotension. The risk is higher in patients with other multiple allergies or history of a prior reaction to contrast agents. To prevent allergic reactions, high-risk patients should be premedicated with corticosteroids (usually prednisone administered the night prior to and morning of the procedure) and a histamine receptor blocker (diphenhydramine), and a nonionic contrast agent should be used.(6,7)
Renal failure is one of the more common complications of catheterization.(8) Renal dysfunction can occur by several mechanisms, including direct contrast-induced nephropathy, dehydration, renal atheroemboli, and hypotension with renal hypoperfusion. Contrast-induced nephropathy is likely the most common cause; it results in a transient rise in the creatinine concentration of more than 1.0 mg/dL in at least 5% of patients undergoing catheterization.(9) Patients with diabetes or preexisting renal insufficiency carry the highest risk.(10) In the setting of contrast-induced nephropathy, the creatinine usually begins to rise 24–48 hours after the procedure, peaks within 3–5 days, and typically returns to baseline within 7 days. Notably, fewer than 1% of patients with contrast-induced renal dysfunction go on to require hemodialysis.(10) Strategies to prevent contrast nephropathy include administering and maintaining adequate hydration, limiting the dose of contrast, and possibly using agents such as acetylcysteine in high-risk patients.(8,9) (The topic of contrast-induced nephropathy was covered in a previous WebM&M commentary.)
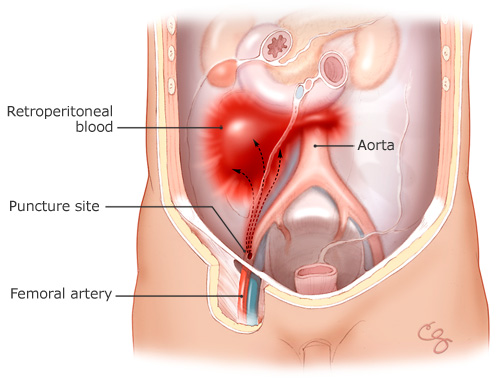
This unfortunate case highlights one of the most common complications of this widely performed procedure. Retroperitoneal hematomas result from bleeding from the femoral artery (which is cannulated during the procedure with a large sheath) into the retroperitoneal space (Figure). The bleeding is typically from arterial puncture above the inguinal ligament or inadequate hemostasis after the procedure but may also occur spontaneously from the anticoagulation used for PCI. Diagnostic catheterizations in the past were typically performed after administration of variable amounts of heparin. In current practice, many of these diagnostic catheterizations are performed without administration of anticoagulant therapy. Anticoagulant therapy used during PCI varies depending on the operator and the clinical situation but can include high doses of unfractionated heparin, enoxaparin, the direct thrombin inhibitor bivalirudin (Angiomax), and platelet glycoprotein IIb/IIIa inhibitors (potent antiplatelet agents). The half-life of these agents varies from between 20 minutes to 12 hours or more. The major determinant of successful ultimate hemostasis is the quality of the initial puncture, which depends primarily on operator skill and experience. Typically, at the end of cardiac catheterization, the sheath in the femoral artery is removed, and hemostasis is achieved from one of several methods. Manual compression of the artery is the traditional method and is easy to perform, but it is associated with the longest period of bed rest, considerable patient discomfort, and a low but definite complication rate. Vascular closure devices (mechanical and biochemical devices that help "seal" the artery) were developed in an attempt to reduce bed rest time, improve patient comfort, and perhaps lower the complication rate associated with manual compression. However, their use may actually increase the risk of local vascular complications with diagnostic catheterization and PCI or the severity of such complications.(11) These depend upon the specific device used and whether the procedure is a diagnostic catheterization or a PCI (12), as well as upon operator expertise. Closure devices may also increase the risk of local infection or endarteritis. Decisions regarding whether to use manual compression or a vascular closure device, as well as which vascular closure device, are complex and must weigh the location of the groin stick, patient body habitus, anticoagulants used, and local expertise with a particular closure device.
In this patient, given the delay in the development of the hematoma, the likely cause of the bleeding was failure of the closure device. Whatever its cause, the diagnosis of retroperitoneal hematoma is initially made on clinical grounds. Symptoms, signs, and laboratory abnormalities that should raise suspicion of retroperitoneal bleed include hypotension, back or flank pain, and an otherwise unexplained drop in hemoglobin. CT scanning can be used to confirm the diagnosis. Retroperitoneal bleeding is treated by aggressive volume resuscitation, correction of coagulopathy, and blood transfusion. Surgery is rarely necessary and is reserved for hypotension unresponsive to volume or for a progressive drop in hemoglobin despite transfusion.(13)
Overall, vascular complications, including retroperitoneal hematomas, account for the highest proportion of complications in cardiac catheterization.(13) Other vascular complications may include acute arterial thrombosis, distal embolization, dissection, pseudoaneurysm, or arteriovenous fistula. A pseudoaneurysm occurs when the arterial puncture site does not adequately seal and can follow manual compression or use of a vascular closure device. Findings suggestive of pseudoaneurysm at the puncture site include pulsatile mass, extreme tenderness, or new bruit. Patients with suspected pseudoaneurysm should undergo Doppler imaging. While smaller pseudoaneurysms can often be managed conservatively, larger pseudoaneurysms will usually be treated with ultrasound-guided occlusion, thrombin injection, surgical repair, or other interventions.
Cardiac catheterization is one of the most commonly performed procedures in medical practice and has low overall complication rates. However, numerous potential life- and limb-threatening complications are possible. For the individual patient, the risk of an adverse event is dependent upon comorbidities, cardiovascular anatomy, demographics, experience of the operator, and the type of procedure being performed. The risk-to-benefit ratio usually favors performance of this procedure as part of the evaluation and treatment of potentially fatal or lifestyle-limiting cardiac disease in appropriately selected patients. Physicians performing cardiac catheterization and those caring for patients who have undergone the procedure must understand the risk of complications and learn how to recognize and treat them. While complications cannot be entirely eliminated, their incidence can be reduced by careful patient selection, preparation, and attention to detail.
Take-Home Points
- Cardiac catheterization is a commonly performed procedure with relatively low overall rates of major and minor complications, but complications do occur and there should be a good clinical indication for the procedure.
- Vascular complications are the most common, although other complications include procedure-associated stroke, MI, and contrast-induced renal failure.
- Retroperitoneal hematomas can result from improper arterial puncture or failure to achieve adequate hemostasis after removal of the femoral artery sheath.
- Arterial puncture closing devices do not necessarily decrease local vascular complications, and patients treated with these devices should undergo the same vigilant monitoring as those treated with manual compression.
- Physicians caring for patients after the procedure should be aware of the common periprocedural complications.
Jose L. Baez-Escudero, MD Fellow in Cardiovascular Disease, Department of Medicine—Section of Cardiology Baylor College of Medicine
Glenn N. Levine, MD Associate Professor of Medicine, Baylor College of Medicine Director, Cardiac Care Unit, Michael E. DeBakey Medical Center
References
1. Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 Update: a report from the American Heart Association Statistics Committee and Stroke Statistics subcommittee. Circulation. 2007;115:e69-e171. [go to PubMed]
2. Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography): developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756-1824. [go to PubMed]
3. Johnson LW, Lozner EC, Johnson S, et al. Coronary arteriography 1984-1987: a report of the Registry of the Society for Cardiac Angiography and Interventions: I: results and complications. Cathet Cardiovasc Diagn. 1989;17:5. [go to PubMed]
4. Laskey WK, Kimmel S, Krone RJ. Contemporary trends in coronary intervention: a report from the Registry of the Society for Cardiac Angiography and Interventions. Catheter Cardiovasc Interv. 2000;49:19-22. [go to PubMed]
5. Segal AZ, Abernethy WB, Palacios IF, et al. Stroke as a complication of cardiac catheterization: risk factors and clinical features. Neurology. 2001;56:975. [go to PubMed]
6. Lasser EC, Berry CC, Talner LB, et al. Pretreatment with corticosteroids to alleviate reactions to intravenous contrast material. N Engl J Med. 1987;317:845-849. [go to PubMed]
7. Wittbrodt ET, Spinler SA. Prevention of anaphylactoid reactions in high-risk patients receiving radiographic contrast media. Ann Pharmacother. 1994;28:236-241. [go to PubMed]
8. Aspelin P, Aubry P, Fransson SG, et al. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491-499. [go to PubMed]
9. Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542. [go to PubMed]
10. Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol. 2002;40:1383-1388. [go to PubMed]
11. Koreny M, Riedmuller E, Nikfardjam M, et al. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291:350. [go to PubMed]
12. Nikolsky E, Mehran R, Halkin A, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. 2004;44:1200. [go to PubMed]
13. Levine GN, Kern MJ, Berger PB, et al. Management of patients undergoing percutaneous coronary revascularization. Ann Intern Med. 2003;139:123–136. [go to PubMed]
Figure
Figure. Retroperitoneal Bleeding from the Femoral Artery. Illustration by Chris Gralapp.